| Product Name | Felodipine |
| Description |
L-type Ca channel blocker |
| Purity | >98% (TLC); NMR (Conforms) |
| CAS No. | 72509-76-3 |
| Molecular Formula | C18H19Cl2NO4 |
| Molecular Weight | 384.25 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 100 mM in ethanol and to 100 mM in DMSO |
| Source | Synthetic |
| Appearance | Light Yellow Solid |
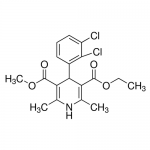
| SMILES | CCOC(=O)C1=C(NC(=C(C1C2=C(C(=CC=C2)Cl)Cl)C(=O)OC)C)C |
| InChI | InChI=1S/C18H19Cl2NO4/c1-5-25-18(23)14-10(3)21-9(2)13(17(22)24-4)15(14)11-7-6-8-12(19)16(11)20/h6-8,15,21H,5H2,1-4H3 |
| InChIKey | RZTAMFZIAATZDJ-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Harmful. May be harmful if inhaled, swallowed or absorbed through skin. Safety Phrases: S22 - Do not breathe dust S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S24/25- Avoid contact with shin and eyes Risk Phrases: R20/21/22 - Harmful by inhilation, in contact with skin and if swallowed R62 - Possible risk of impaired fertility Hazard Phrases: H302 |
| Cite This Product | Felodipine (StressMarq Biosciences, Canada, Cat # SIH-323) |
Biological Description
| Alternative Names | Ethyl methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydro-3,5-pyridinedicarboxylate, Plendil |
| Research Areas | Calcium Channels, Ion Channels, Neuroscience, Voltage-Gated Calcium Channels |
| PubChem ID | 3333 |
| Scientific Background | Felodipine is an L-type Ca2+ channel blocker (1). In isolated rat ventricular myocytes, using a whole-cell patch-clamp technique, it displays a Ki of 11 nM (2). It is a clinically useful antihypertensive agent (3) which attenuates the hypertrophic effect of cyclosporine on transplanted hearts (4). |
| References |
1. Furakawa T., et al. (2005) J Cardiovasc. Pharmacol. 45(3): 241-246. 2. Zahradnikova Z., Minarovic I., Zahradnik I. (2007) J Pharmacol Exp. Ther. 322(2): 638-645. 3. Manzo B.A., Matalka M.S., Ravnan S.L. (2003) Pharmacotherapy. 23(11): 1508-1512. 4. Schwitter J., et al. (1999) J Heart Lung Transplant. 18(10): 1003-1013. |

Reviews
There are no reviews yet.