The Epichaperome in Alzheimer’s Disease: The Effect of Stressors on Protein Networks
The human body faces a variety of stressors throughout life including genetic and environmental factors. Stress is an organism’s response to such stressors. While mild stress can have positive effects on human nervous system, chronic stress can lead to neuronal deficits and neurodegeneration1,2. The hippocampus, which has a major role in learning and memory, is the most vulnerable brain area that undergoes neurodegeneration under chronic stress3. In Alzheimer’s disease (AD), a chronic neurodegenerative disorder, stressors cause imbalances in the connectivity of neural networks, neuronal proteins and protein pathways important for synapse formation and function4.
What is the epichaperome?
Molecular chaperones such as Heat Shock Proteins (HSPs) are activated under stress conditions and play crucial role in protein folding and unfolding as well as in protein degradation and disaggregation. Chaperones, co-chaperones and folding enzymes compose the chaperome which, under cellular stress, forms stable protein networks known as the epichaperome. Previous studies have shown that the epichaperome network is also involved in tumor growth and survival6.
Heat Shock Proteins and Tau Misfolding
HSP90 and HSP70 regulate tau homeostasis. Specifically, the HSP90/co-chaperone complex regulates tau folding and the HSP70/CHIP (Carboxyl terminus of HSP70 Interacting Protein) co-chaperone complex mediates tau degradation5. In tauopathies like Alzheimer’s, tau homeostasis is disrupted, leading to its hyperphosphorylation and the accumulation of intracellular aggregates.
How is the epichaperome involved in Alzheimer’s disease?
A study published in Nature Communications shows how the switch of the chaperome into the epichaperome disturbs protein networks, leading to protein connectivity-based dysfunction (PCBD) and cognitive decline. Authors, at the Memorial Sloan Kettering Cancer Center, proposed that AD is a protein connectivity–based disorder.
The researchers compared PS19 tau transgenic mice, which express human P301S mutant tau, to wild-type mice, and found that the PS19 mouse brains contained more epichaperomes than their wild-type counterparts. Interestingly, the epichaperomes formed prior to the development of tau pathology, which suggests they may enable the formation of tau tangles.
Similar results were seen when the researchers compared brain tissues from AD patients to normal of the same age. They found a significantly higher number epichaperomes in the AD brain tissues.
Effects of Epichaperome Formation
The researchers used beads to capture the epichaperome and its interactome and analyze protein-protein interaction (PPI) networks. They found that epichaperome formation induced synaptic dysfunction. They confirmed this by overexpressing tau in N2a (mouse neuroblastoma) cells, which induced imbalances in synaptic protein networks. Several pathways became dysregulated due to defective long-term potentiation (LTP).
PU-AD Targets the Epichaperome
Dr. Chiosis’ research group at the Memorial Sloan Kettering Cancer center developed the synthetic purine-scaffold inhibitor PU-H71, which targets HSP90 within the epichaperome in cancer cells6.
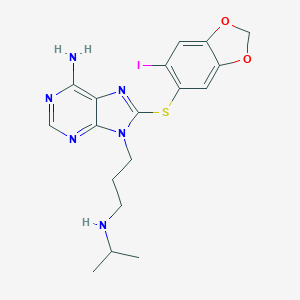
PU-H71 Chemical Structure (https://pubchem.ncbi.nlm.nih.gov/compound/pu-h71)
In this study, the same group developed a chemical probe called PU-AD which inhibits the epichaperome, returning the network connectivity and functional imbalances to normal levels. PU-AD, like PU-H71, inhibits HSP90, enhancing the degradation of mutated or hyperphosphorylated tau.
Researchers treated PS19 mice with PU-AD and used chemical chaperomics to detect and analyze synaptic protein network dysfunctions. The drug delivery led to a reduction in protein connectivity dysfunctions and the mice demonstrated improved memory and longer survival without any side effects. The results indicated the effectiveness and safety of PU-AD which is now being tested in human patients.
StressMarq’s HSP90, HSP70/HSC70 and HSP110 antibodies were used for Western Blot in epichaperome analysis of mouse and human brain samples and N2a cells overexpressing human tau.
REFERENCES
- McEwen, B. S. The brain on stress: toward an integrative approach to brain, body, and behavior. Perspect. Psychol. Sci. 8, 673–675 (2013).
- Arnsten, A. F. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat. Neurosci. 18, 1376–1385 (2015).
- Bartsch, T. & Wulff, P. The hippocampus in aging and disease: from plasticity to vulnerability. Neuroscience 309, 1 –16 (2015).
- Querfurth, H. W. & LaFerla, F. M. Alzheimer’s disease. N. Engl. J. Med. 362, 329–344 (2010).
- Thompson, A. D. et al. Analysis of the tau-associated proteome reveals that exchange of Hsp70 for Hsp90 is involved in tau degradation. ACS Chem. Biol. 7, 1677–1686 (2012).
- Pillarsetty, N. et al. Paradigms for precision medicine in epichaperome cancer therapy. Cancer Cell 36, 559–573 (2019).
- Inda, M. et al. The epichaperome is a mediator of toxic hippocampal stress and leads to protein connectivity-based dysfunction. Nature Communications 11, 319 (2020).

Leave a Reply