Modelling Alzheimer’s Disease in Cells and Animals
Alzheimer’s disease is complex with multiple mechanisms leading to neuronal loss and death. This complicates the development of treatments – certain drugs or interventions may effectively target some aspects of the disease but not others. There are various in vitro and in vivo models that help researchers understand AD pathogenesis and conduct preclinical testing. An ideal model should mimic the development of AD demonstrating the progressive neurodegeneration as well as amyloid plaque and neurofibrillary tangle formation.
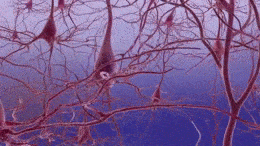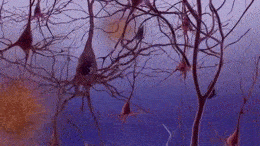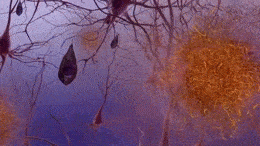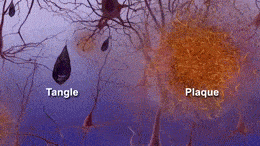
Formation of neurofibrillary tangles and beta-amyloid plaques. (https://creativecommons.org/licenses/by-sa/3.0)
Before selecting a model, the scientists should consider the aspect of the disease they intend to study; most of the models have been designed to reproduce one or more pathological, biochemical or behavioral characteristics of Alzheimer’s disease1. Sporadic AD, which is the most prevalent form, is influenced by genetic and environmental risk factors. Studies suggest that lifestyle age-related risk factors such as stress, smoking, alcohol consumption, high blood pressure, and diabetes play a critical role in the disease. Most animal models have been designed to mimic the genetic form of AD and they do not exhibit all AD pathological features. So far there are no models for sporadic AD2.
Cellular Models of Alzheimer’s Disease
In vitro models have been widely used in AD research to investigate the disease pathology and progression as well as in drug screening. Cell lines used in AD research include primary neuronal cultures, cancer cell lines and induced pluripotent stem cells (iPSCs). Different cellular models are used to study different aspects of the disease. Cellular models cannot represent the complex environment of the human brain, which includes non- neuronal cells that might play a significant role in AD pathology as well as the interactions between neurons. Nevertheless they can be valuable tools in AD studies and drug discovery3,4.
Primary Cell Lines
Primary cell lines can be cultured from transgenic animal or human patients. Immortal rat hippocampal cell lines have been used extensively as the hippocampus is the most affected area in AD. Primary cortical cultures have also been used to examine the effect of amyloid beta oligomers on neuron function and apoptosis. Human embryonic kidney cells (HEK293) are used to model tau-related effects and can be easily transfected with tau. Primary cultures can also be transfected with APP and can give useful information on the role of amyloid beta oligomers5.
Cancer Cell Lines
Neuroblastoma (SH-SY5Y)
Human neuroblastoma (SH-SY5Y) cells are isolated from a cancer of immature neuronal cells in the embryonic stage and they can be differentiated into different phenotypes depending the growth factor6. In AD research, SH-SY5Y cells are differentiated into neuron-like cells with similar morphology to mature neurons. These cells are then exposed to toxic amyloid beta oligomers that lead to neurodegeneration. This model has become very popular in neurodegeneration studies; however it does not fully reproduce the aspects of the disease due to the interference of the cancer genes3.
iPSC Models of Alzheimer’s Disease
2D iPSC Neuron Cultures
Cell culture in a petri dish. kaibara87 / CC BY (https://creativecommons.org/licenses/by/2.0)
Pluripotent stem cells are capable of differentiating into different cell types and can be generated from reprogramming mature somatic cells. Induced pluripotent stem cell (iPSC) technology can be used to model Alzheimer’s disease, allowing for the study of individual neuronal cells obtained from FAD or SAD patients. In 2011 researchers produced iPSCs from primary human fibroblast cells of FAD patients with PS1 and PS2 mutations, which showed increased levels of amyloid beta peptides, a major hallmark in AD pathology6. In 2012, another research group generated iPSCs from FAD and SAD patients; the iPSC-derived neurons showed significantly higher levels of amyloid beta and phosphorylated tau, but could not recapitulate amyloid plaques and tau tangles7.
Progress in iPSC technology allows the generation of patient-specific neurons which can be applied for personalized medicine8. The main limitation in the use of iPSC models is the lack of maturity and aging characteristics as AD is an age-related disease9.
3D iPSC Cerebral Organoids
One of the recent advances in neuroscience is the generation of organoids, produced from human iPSCs. Cerebral organoids, also called mini- brains, are in vitro self- organized 3D structures similar to the human brain and are a valuable scientific tool in neurodegenerative disease modelling. In Alzheimer’s research, 3D brain organoids can reproduce complete amyloid beta deposits, tau tangles and neurodegeneration. Their lack in cellular diversity, low synaptic activity and incomplete immune system are the most critical limitations of these 3D models10.
Animal Models of Alzheimer’s Disease
The majority of experimental models used in AD research are animal models. Various species have been used such as Drosophila melanogaster, Caenorhabditis elegans, Danio rerio, non –human primates and rodents. Transgenic mice expressing human genes are commonly used since they reproduce AD hallmarks, have similar anatomy to humans, and are low cost and easy to manipulate.
In the last several decades, various mouse models have been generated to produce the amyloid beta plaques and the neurofibrillary tangles associated with neurodegeneration. So far, many transgenic mouse models have been developed with mutations in the APP or presenilin (PSEN1, PSEN2) or combinations (3×Tg, 5×Tg). However, these models mimic the genetic form of AD and they cannot give information on the sporadic form2. Genes such as APOE e4 and TREM-2 enhance the risk of sporadic AD, but also many lifestyle risk factors may play a role. Mouse models can be divided into different groups depending the gene mutations they carry and their characteristics.
Transgenic Models of Alzheimer’s Disease11,12,13
1. APP Single Transgenic mice
The APP transgenic mice are generated by overexpressing the APP gene. They exhibit amyloid beta plaques and cognitive deficits but fail to develop neurofibrillary tangles.
2. Tau Transgenic Mice
These models express human tau protein and demonstrate intraneuronal tau tangles and behavioral and motor deficits, but do not develop amyloid plaques. The model rTg4510 expresses high levels of human tau containing the P301L mutation and develops progressive neurofibrillary tangles, neuronal loss and behavioral impairments.

3. APP/Tau Double Transgenic Mice
Double transgenic mice express the APP and Tau proteins and exhibit amyloid beta plaques, intraneuronal tau tangles, and motor deficits.
4. APP/PSEN1 Double Transgenic Mice
The mice express APP and PSEN1 mutations and exhibit only amyloid beta plaques.
5. APP/Tau/PSEN1 Triple Transgenic Mice (3xTg-AD)
These models contain mutations in APP, MAPT and PSEN1 and display both plaques and tangles. The animals exhibit more severe pathology compared to single and double transgenic models.
6. Five Transgenic Mice (5xFAD)
This widely used amyloid beta model carries 5 AD mutations into a single transgenic line and shows an early amyloid pathology (2 months) and more severe neuropathology compared to other models. It lacks neurofibrillary tangles.
APP Knock-In Mice
Knock-in mouse models generate higher levels of amyloid beta without overexpressing APP. Mice show typical amyloid beta pathology, neuroinflammation and memory impairment.
APOE and TREM2 Models
APOE and TREM2 are significant risk factors for sporadic AD. Transgenic, knock-out and knock-in mice expressing human APOE have been generated. New knock-out lines of TREM2 have been generated to investigate the mechanisms of the gene associated with SAD.
Recombinant Protein Models of Alzheimer’s Disease
Amyloid Beta Oligomers
Several studies have demonstrated that applying exogenous soluble amyloid beta oligomers and fibrils into animal brains or cultured brain slices causes synaptic and cognitive dysfunction as well as abnormal tau phosphorylation and neuronal loss. In non-human primates like macaques, intracerebroventricular injections of amyloid beta oligomers induce synapse loss, microglial and astrocyte activation, tau phosphorylation, and NFT formation14. This new model, which is similar to humans, reproduces pathological features of AD and it may be useful in future drug development.
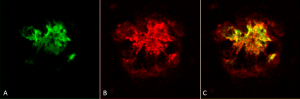
Immunohistochemistry analysis using Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (catalog# SPC-506). Composite. Courtesy of: Dr. Elizabeth Head, University of California, Irvine.
Tau Preformed Fibrils (PFFs)
Tau preformed fibrils (PFFs) can induce aggregation of tau in both cultured cells and animals by recruiting soluble monomers to form insoluble fibrils. Tau preformed fibrils can be used as tools to mimic Alzheimer’s pathophysiology in the brain and they can induce pathology in a short period compared to transgenic animals. Tau PFFs have been injected in the hippocampus of tau transgenic mice (P301L) where they seed tau aggregation and induce tau pathology in the brain15.
StressMarq Biosciences offers a variety of tau and amyloid beta Monomers, Oligomers and Pre-formed Fibrils to help researchers investigate tau aggregation, one of the main hallmarks of Alzheimer’s disease. We also offer a variety of Amyloid Beta and Tau Antibodies, as well as other products for Alzheimer’s disease research.
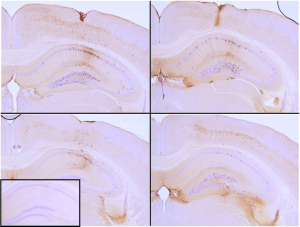
Immunohistochemistry analysis of P301L mouse hippocampus injected with K18 P301L tau PFFs (catalog# SPR-330) shows seeding of tau pathology at injection site nine weeks post-injection. Experiments performed at reMYND N.V.
REFERENCES
- Modeling Alzheimer’s disease: from past to future. Saraceno C. et al. (2013) Front Pharmacol. 4:77.
- Can mouse models mimic sporadic Alzheimer’s disease? Foidl BM. et al. (2020) Neural Regen Res 15(3):401-406
- An overview of in vitro research models for Alzheimer’s disease, Carolindah MN et al . (2013) J of TESMA Regenerative Research 2(2): 8-13
- Alzheimer’s disease: Experimental models and reality, Drummond E. et al.(2017) Acta Neropathol. 133(2):155-175
- General overview of neuronal cell culture. Gordon J. et al. (2013) Methods Mol Biol. 1078:1–8.
- Modeling familial Alzheimer’s disease with induced pluripotent stem cells, Yagi T. et al. (2011) Human Molecular Genetics 20(23):4530–4539
- Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells, Israel MA et al. (2012) Nature 482(73840): 216 -220
- Translation of Human-Induced Pluripotent Stem Cells: From Clinical Trial in a Dish to Precision Medicine. Sayed N. et al. (2016) J Am Coll Cardiol. 67(18):2161–2176.
- Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications. Doss MX. et al. (2019) Cells.8(5):403.
- Brain organoids: a next step for humanized Alzheimer’s disease models? Gerakis, Y. et al. (2019) Mol Psychiatry 24: 474–478.
- Practical considerations for choosing a mouse model of Alzheimer’s disease, Jankowsky JL. et al. (2017) Mol. Neurodegeneration 12: 89
- Overview of transgenic mouse models for Alzheimer’s disease, Myers A. et al. (2019) Current Protocols in Neuroscience 89:81
- Mouse models of Alzheimer’s disease, Hall AM. et al. (2011) Brain Research Bulletin 88(1): 3-12
- Alzheimer’s disease-like pathology induced by amyloid beta oligomers in nonhuman primates, Forny-Germano L. et al. (2014) The Journal of Neuroscience 34(41): 13629-13643
- Intracerebral injection of preformed synthetic tau fibrils initiates widespread tauopathy and neuronal loss in the brains of tau transgenic mice. Peeraer E. et al. (2015) Neurobiology of disease 73: 83-95.


Leave a Reply