Tau Induces Astrocyte Senescence in Alzheimer’s Disease
Neurodegenerative tauopathies like Alzheimer’s disease (AD) present insoluble tau filaments accumulating in the brain1,2,3. Tau stabilizes microtubules in the neurons. However, hyperphosphorylation and misfolding disrupts this process3. Misfolding can also spread cell to cell, further propagating neurotoxic pathology3. Understanding how tau proteins impact neurons and glial cells can help researchers develop novel therapeutic targets.
Astrocytes are the most abundant cell type in the brain, and dysregulation has a role in tauopathies4. For example, senescence of astrocytes exhibits pathology through pro-inflammatory cytokines4. Researchers at the National Institute of Health used our tau monomers and pre-formed fibrils to study its effects on astrocyte senescence. This research shows a novel mechanism of extracellular tau directly impacting neurodegeneration.
What is SASP?
When cells experience certain stressors or undergo sufficient divisions, they enter cellular senescence6. This leads to the upregulation of cell cycle inhibitors and senescence-associated beta galactosidase, as well as various cytokines, and proteases6. This change in expression is called senescence-associated secretory phenotype (SASP)4,6.
Astrocyte senescence is observed in AD patients and brain injuries, and have been suspected to promote inflammation through SASP5,6. This evidence increases the value targeting cellular senescence in neurodegeneration.
Truncated Tau Fragment dGAE
StressMarq’s selection of tau pre-formed fibrils (PFF) help researchers further our understanding of tauopathies. In this study, Ungerleider, et al. used our active truncated dGAE AA297-391 tau monomer (catalog# SPR-444) and dGAE AA297-391 tau PFFs (catalog# SPR-461) to test in vitro effects on astrocyte senescence. Human astrocytes were treated with extracellular tau monomers, PFFs or both.
The truncated 95-amino acid form of tau (dGAE) assembles into paired helical filaments that makeup neurofibrillary tangles in AD brains1. Furthermore, this assembly occurs independently of additives, such as heparin5,7. Researchers postulate that dGAE tau closely models pathology of tauopathy.
TEM of active human recombinant truncated tau fragment (AA297-391) (dGAE) protein preformed fibrils (SPR-461).
Tau Induces Senescence in Astrocytes
Results show that compared to no treatment, extracellular tau significantly increases senescence in astrocytes. This is demonstrated by the increase of SA- β-gal, p21 mRNA and p16 protein levels, biomarkers known to upregulate during cellular senescence5.
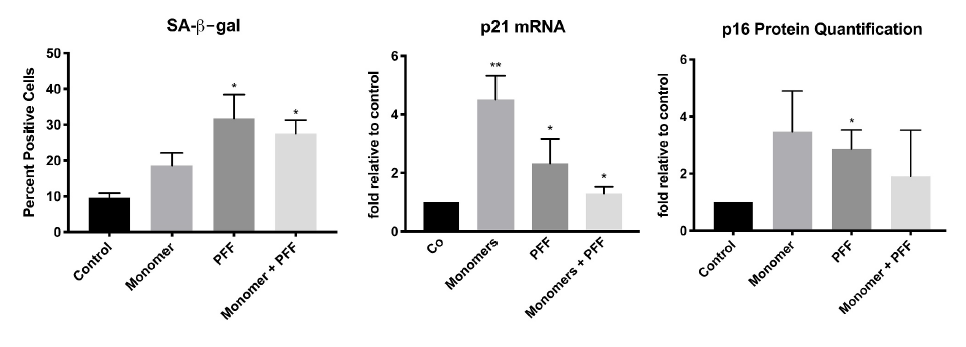
Presence of tau induces cellular senescence in human astrocytes. Cells were treated with tau monomers, PFFs or both with (total 0.1 ug/mL) for 24 H. Following, cells were allowed to recover for 48H prior to RNA/protein extraction or SA-β-gal staining. Taken from Ungerleider, et al. 2021.
Astrocytes Demonstrate SASP in Presence of Tau
Using the same treatments as above, the effect of tau on the pro-inflammatory response in astrocytes was investigated. Following treatment of tau, there was increased in IL-1β, IL-8, NOS2, and TNFα mRNA levels. These results indicate astrocytes undergo SASP in response to extracellular tau, subsequently undergoing inflammation and oxidative stress.
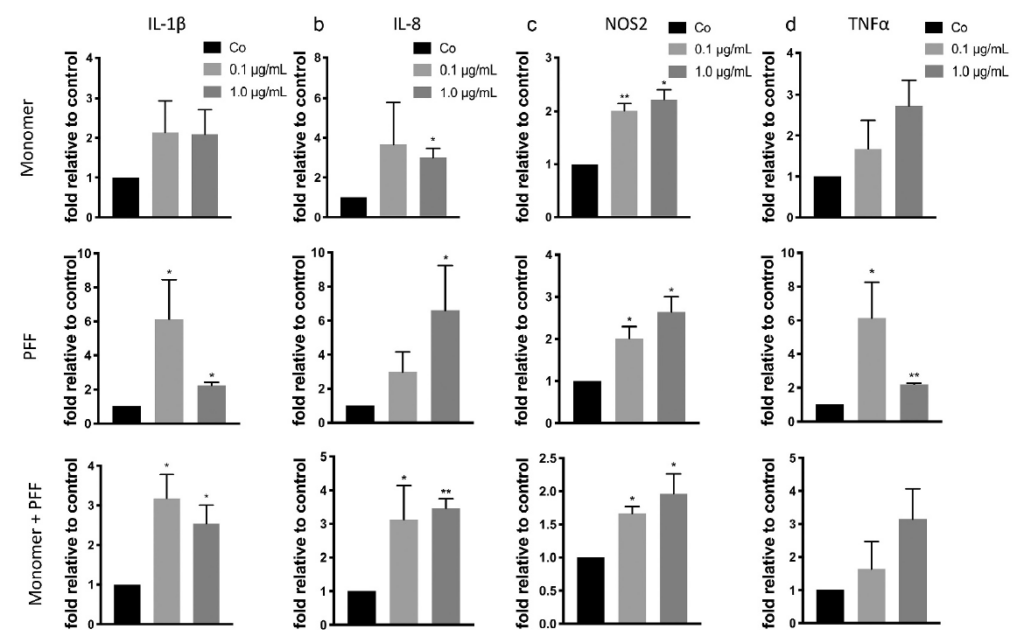
Presence of tau induces inflammation and oxidative stress in human astrocytes. Cells were treated with tau monomers, PFFs or both for 24 H. Following, cells were allowed to recover for 48H prior to RNA extraction. Expression was measured by qRT-PCR. Taken from Ungerleider, et al. 2021.
Further Research into the Role of Tau in Neurodegenerative Disease
Tau misfolding is the key player in many neurological diseases. This research highlights a new implication of aggregation in the brain. Here, tau proteins impact neurotoxicity by promoting astrocyte senescence. Subsequent SASP can progress neurodegeneration with inflammatory markers and oxidative stress.
This insight on tau-induced senescence of brain cells can be pursued in future studies of tauopathies. Novel therapeutic targets against senescing astrocytes can be a viable option for future treatments of these diseases.
References
- Soluble tau aggregates, not large fibrils, are the toxic species that display seeding and cross-seeding behavior, Ghag G et al, Protein science: a publication of the Protein Society. 2018 Nov; 27(11), 1901–1909.
- Tau pathology and neurodegeneration, Spillantini MG et al, Lancet Neurol. 2013 Jun; (6):609-22.
- Spreading of α-synuclein and tau: a systematic comparison of the mechanisms involved, Vasili E et al, Frontiers in molecular neuroscience. 2019 April 5; 12: 107.
- p53 isoforms regulate astrocyte-mediated neuroprotection and neurodegeneration, Turnquist C et al, Cell death and differentiation. 2016 Sept 1; 23(9), 1515–1528.
- Astrocyte senescence and SASP in neurodegeneration: tau joins the loop. Ungerleider K et al, Cell Cycle. 2021 Apr 5; 1-13.
- Radiation-induced astrocyte senescence is rescued by Δ133p53, Turnquist C et al, Neuro-oncology. 2019 Mar 18; 21(4), 474–485.
- Alzheimer’s Disease-like paired helical filament assembly from truncated tau protein is independent of disulfide crosslinking, Al-Hilaly YK et al, J Mol Biol. 2017 Nov 24;429(23):3650-3665.


Leave a Reply