The Role of Amyloid Beta Oligomers in Alzheimer’s Disease
Alzheimer’s disease (AD) is characterized by a progressive loss of memory and executive function, which is attributable to synaptic damage and neurodegeneration within various regions of the brain. It represents the most common cause of dementia worldwide and AD incidence is predicted to rise with an aging population. For decades, the amyloid-β (Aβ) protein has been the central focus of Alzheimer’s disease research, largely since the brains of AD patients contain senile plaques in which Aβ is the major constituent. However, while senile plaques were once considered to be responsible for Alzheimer’s disease pathology, this is no longer the case1,2. Instead, a growing body of evidence points toward Aβ oligomers being the major toxic species of Aβ, highlighting their potential for therapeutic targeting.
The amyloid cascade hypothesis has fallen from favor
Under normal conditions, Aβ peptides ranging from 37 to 49 residues in length are sequentially cleaved from the amyloid precursor protein (APP) by β- and γ-secretase enzymes, before being released from cells and rapidly degraded. Dysregulation of this process leads to Aβ peptides accumulating and forming amyloid fibrils, which give rise to senile plaques. Aβ 40 and Aβ 42 are the main constituents of the plaques, with Aβ 42 being the more neurotoxic of the two isoforms3. In 1992, it was suggested that senile plaques are responsible for the formation of neurofibrillary tangles and subsequent neuronal cell death, a phenomenon termed the amyloid cascade hypothesis1. But, over the years, several significant observations have led researchers to doubt this theory. These include the poor correlation between Alzheimer’s disease onset and plaque burden; the finding that neuronal cell death occurs in brain regions devoid of plaques; and the discovery that Aβ plaques are present in cognitively normal individuals. Additionally, some patients presenting with symptoms of Alzheimer’s disease lack pathological changes in their brains, indicating that larger aggregates are not the cause of neurodegeneration4.
Uncovering a role for Aβ oligomers in AD pathogenesis
Now, the amyloid cascade hypothesis has been superseded by the oligomer cascade hypothesis, which proposes that oligomers, rather than senile plaques, are the initiating pathologic agents in Alzheimer’s disease2. Oligomers are small spherical structures, ranging from approximately 3 to 10 nm in diameter, which consist of multiple Aβ peptides that are non-covalently assembled into clusters4,5. They are noticeably increased in the early stages of AD, when they localize at or within the neuronal synapses, and have been shown to exert their toxic effects via numerous experimental approaches6. For example, Aβ oligomers generated in vitro have proven toxic to primary cultures of murine cortical neurons, while oligomers extracted directly from Alzheimer’s disease brains have been shown to impair synapse structure and function7,8. More recently, studies performed in iPSC-derived human neurons have demonstrated several proteins to be differentially regulated in response to oligomer challenge, while investigations using human AD and murine brain tissues have identified synaptic proteins to which oligomers attach, prompting efforts to determine how they might be exploited as therapeutic targets6,9.
Supporting Alzheimer’s disease research
StressMarq offers an extensive selection of products to support Alzheimer’s disease research, including Aβ monomers (catalog# SPR-485), oligomers (catalog# SPR-488), and pre-formed fibrils (catalog# SPR-487). Our Aβ oligomers are generated from Aβ peptide 1-42, pre-treated with 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) using published methods. They exhibit a globular conformation when observed under transmission electron microscopy (TEM) and atomic force microscopy (AFM) and have a unique dimer/trimer and oligomer signal on a Western Blot with an anti-amyloid beta antibody. Importantly, we have generated data showing our Aβ oligomers to be toxic to primary rat cortical neurons in a dose-dependent manner.
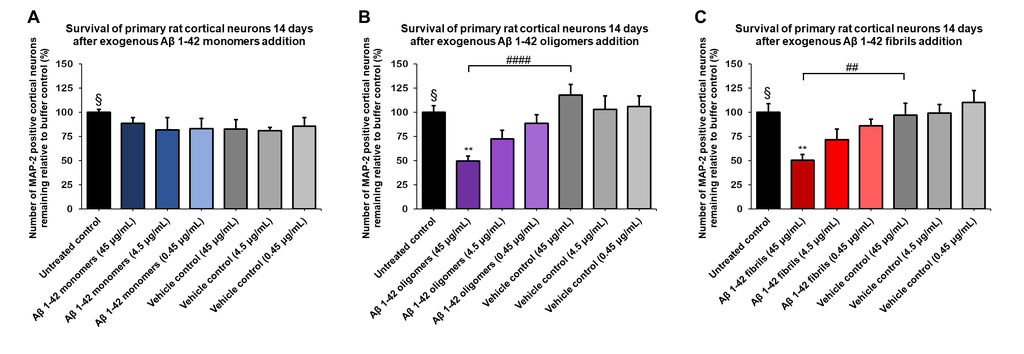
Amyloid beta 1-42 oligomers (SPR-488) and fibrils (SPR-487) show a dose-dependent toxicity to primary rat cortical neurons, but not monomers (SPR-485). Survival of rat primary cortical neurons 14 days after treatment with different concentrations of (A) monomers, (B) oligomers or (C) fibrils quantified by MAP2 positive neurons and expressed as a percentage of control. Fibrils and respective vehicle controls were initially sonicated in a Bioruptor. Test conditions were run in the same plate as untreated control and vehicle controls, which consisted of buffer without amyloid beta 1-42 protein. Data expressed as mean +/- s.e.m. (n=6). A global analysis of the data was performed using a one-way ANOVA followed by Dunnett’s test; ** p<0.01 stats vs control; ## p<0.01, #### p<0.0001 stats vs vehicle control. § represents untreated control condition.
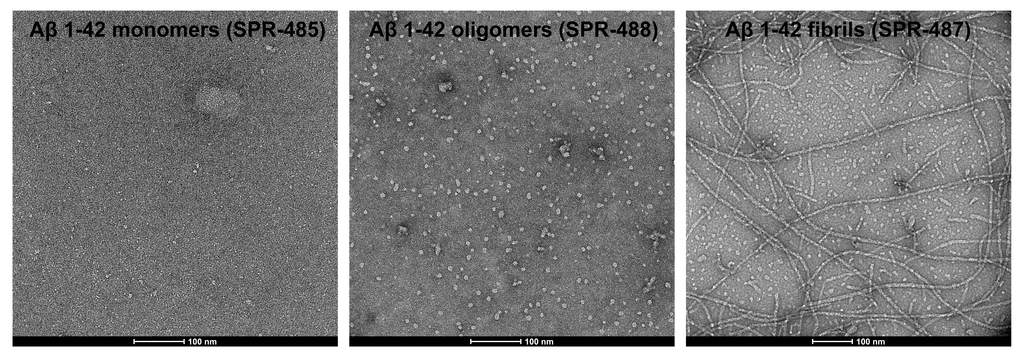
TEM of amyloid beta 1-42 monomers (SPR-485, left), oligomers (SPR-488, middle) and fibrils (SPR-487, right). Negative stain transmission electron microscopy images acquired at 80 Kv on carbon coated 400 mesh copper grids using phosphotungstic acid and uranyl acetate stain. Scale bar = 100 nm.
StressMarq also offers antibodies to Amyloid Beta, specifically an Amyloid Oligomers (A11) Antibody (cat# SPC-506) and an Amyloid Fibrils (OC) Antibody (cat# SPC-507). Both have been validated for Western Blot, IHC, ICC/IF and Immunoprecipitation.
References
- Alzheimer’s disease: the amyloid cascade hypothesis, Hardy JA and Higgins GA, Science. 1992;256(5054):184-185
- Amyloid β-protein oligomers and Alzheimer’s disease, Haydon EY and Teplow DB, Alzheimers Res Ther. 2013;5(6):60
- Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer’s Disease, Kametani F and Hasegawa M, Front Neurosci. 2018;12:25
- The Role of Amyloid-β Oligomers in Toxicity, Propagation, and Immunotherapy, Sengupta U et al, EBioMedicine. 2016 Apr; 6: 42–49
- Kinetic diversity of amyloid oligomers, Dear AJ et al, Proc Natl Acad Sci U S A. 2020;117(22):12087-12094
- Amyloid Beta Oligomers Target to Extracellular and Intracellular Neuronal Synaptic Proteins in Alzheimer’s Disease, Ding Y et al, Front Neurol. 2019;10:1140
- Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils, Ahmed M et al, Nat Struct Mol Biol. 2010;17(5):561-567. doi:10.1038/nsmb.1799
- Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory, Shankar GM et al, Nat Med. 2008;14(8):837-842.
- Oligomeric amyloid-β induces early and widespread changes to the proteome in human iPSC-derived neurons, Sackmann C and Hallbeck M, Sci Rep. 2020;10(1):6538


Leave a Reply