C-Terminally Truncated Alpha Synuclein in Human Erythrocytes
Neurodegenerative diseases, including Parkinson’s disease, are becoming increasingly prevalent in the global population. At the molecular level, Parkinson’s disease (PD) is characterized by the formation of intraneuronal inclusions known as Lewy bodies (LBs). One of the major components of these LBs is the alpha synuclein protein, which initially aggregates into neurotoxic fibrils and subsequently forms larger fibrillar aggregates. Furthermore, Lewy body pathology can spread between interconnected brain regions, and is correlated with Parkinson’s disease progression.
A key characteristic of the alpha synuclein found within LBs is the post-translational phosphorylation of the Serine 129 residue. Compelling evidence also supports the role of other post-translational modifications (PTMs) in pathological conditions. Pathological inclusions in Parkinson’s disease have now been found to contain C-terminally truncated forms of alpha synuclein, which exhibit a higher propensity for fibrillization when compared with the full-length protein. The abundance of this protein in both the central nervous system and peripheral blood is further described in new findings from Amagai et al., which depicts the first observation of C-terminally truncated forms of alpha synuclein in erythrocytes.
In this recent report, peripheral erythrocyte alpha synuclein was employed as a biomarker for PD pathology, and levels of the total, aggregated, and phosphorylated forms of alpha synuclein were subsequently found to be altered with disease progression. This study lays the groundwork for understanding whether C-terminally truncated erythrocyte alpha synuclein may be a potential therapeutic target for neurodegenerative diseases.
Antibodies against C-terminal alpha synuclein fail to detect two erythrocyte protein bands
To begin the investigation into which alpha synuclein protein forms are present in human erythrocytes, blood samples were obtained from healthy volunteers and separated using centrifugation into different components, including erythrocytes and plasma. As alpha synuclein exhibits conformational plasticity, high-resolution clear native polyacrylamide gel electrophoresis (PAGE) was used to characterize the native proteins based on size and conformation. Following separation, five different antibodies targeting different regions of alpha synuclein were used in Western Blotting experiments. Four alpha synuclein antibodies targeting the N-terminal and central portion of the protein were able to recognize all four separated bands. Two antibodies that exclusively targeted the C-terminal of alpha synuclein were unable to bind two out of four total protein bands — annotated as bands ‘A’ and ‘B’ — in the study. This implied that the ‘A’ and ‘B’ alpha synuclein protein bands lacked the C-terminal region.
Excluding the involvement of acetylation on mobility shifts
Next, it was important to ascertain whether the ‘A’ and ‘B’ alpha synuclein protein bands lacked the C-terminal region or if another common PTM caused changes in the band pattern. One of the most frequent PTMs in eukaryotic proteins is N-α-acetylation. N-terminal acetylation of alpha synuclein has been shown to alter lipid-dependent aggregation behaviour, directly impacting in vitro aggregation by modifying the structural properties of fibrillar aggregates. Indeed, PTMs such as acetylation can change the protein band pattern by causing mobility shifts.
The molecular forms of alpha synuclein were analyzed by separating erythrocyte alpha synuclein using size exclusion chromatography (SEC). Comparison of erythrocyte alpha synuclein with StressMarq’s N-Terminal Acetylated Alpha Synuclein Monomers (catalog# SPR-331) was an important step in determining that the additional mobility bands appearing in the erythrocyte alpha synuclein protein were not produced by N-terminal acetylation of alpha synuclein giving rise to mobility changes. The results proved that the ‘A’ and ‘B’ alpha synuclein protein bands belonged to a distinct protein species — the C-terminally truncated form of alpha synuclein. At this point in the study, the focus shifted to mass spectrometry analysis to confirm the primary amino acid sequence of the protein.
For mass spectrometry (LC-MS/MS) experiments, C-terminally truncated alpha synuclein was purified from erythrocytes using antibody immunoprecipitation followed by protein separation with SDS-PAGE. Both full-length and C-terminally truncated forms of the protein were shown to be present in erythrocytes. The mass spectometry data confirmed that the C-terminal truncation was located between amino acid residues Y133 and Q134.
Circulating extracellular vesicles contain C-terminally truncated alpha synuclein
To conclude the study, the researchers examined the plasma component of the collected blood. Extracellular vesicles (EVs) isolated from the blood have attracted much attention as circulating biomarkers for disease discovery. Erythrocyte-derived extracellular vesicles — small fragments released from red blood cells — play an important role in the dissemination of pathogenic protein aggregates in various neurodegenerative diseases, including Parkinson’s disease. It was conlcuded that the EVs derived from erythrocytes contained full-length alpha synuclein as well as C-terminally truncated alpha synuclein.
Investigating alpha synuclein PTMs using StressMarq’s neurodegenerative protein constructs
In this study, StressMarq’s N-Terminal Acetylated Alpha Synuclein Monomers (catalog# SPR-331) were critical in ascertaining that the alpha synuclein found in erythrocytes were modified by C-terminal truncation and not caused by mobility shift changes induced by the most common PTM (N-terminal acetylation). The insights uncovered in this study highlight the versatile functionality of StressMarq’s dedicated range of alpha synuclein protein constructs.
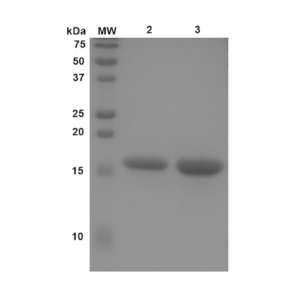
Figure 1. Western Blot analysis of StressMarq’s N-Terminal Acetylated Alpha Synuclein Monomers (catalog# SPR-331).
Summary
Alpha synuclein is abundant in both the central nervous system and peripheral blood. By monitoring the alteration of total, aggregated, and phosphorylated alpha synuclein levels, it may be used as a biomarker to track Parkinson’s disease progression. Evidence is building to support the importance of its C-terminally truncated forms within human pathological inclusions. Previously only reported in the brain, the recent findings by Amagai et al. reveal the first identification of C-terminally truncated alpha synuclein in erythrocytes and corresponding erythocyte-derived extracellular vesicles. These findings ultimately implicate C-terminally truncated erythrocyte alpha synuclein as a new therapeutic target for neurodegenerative disease treatment.
Related StressMarq products
StressMarq’s extensive portfolio of alpha synuclein, tau, and amyloid beta proteins for neurodegenerative disease research includes Alpha Synuclein N-Terminal Acetylated Pre-formed Fibrils (catalog#SPR-332), and newly released Alpha Synuclein pSer129 Pre-formed Fibrils (catalog#SPR-521) & Monomers (catalog# SPR-520). Visit our website for more information, including the latest scientific publications using our specialized protein constructs.
References
- C-terminally truncation is a prominent post-translational modification of human erythrocyte α-synuclein. Amagai, R., et al. The Journal of Biochemistry. 2024.
- Physiological C-terminal truncation of α-synuclein potentiates the prion-like formation of pathological inclusions. Sorrentino, Z. A. et al. The Journal of Biological Chemistry. 2018.
- N-Terminal acetylation of α-Synuclein slows down its aggregation process and alters the morphology of the resulting aggregates. Bell, R., et al. Biochemistry. 2022.


Leave a Reply