Microglial Activation in Alzheimer’s Disease
Discovered more than 100 years ago in 1919, microglia are the resident macrophages of the brain, and are involved in first-line innate immunity – including phagocytosis and clearance of pathological protein aggregates. Microglia use their highly branched (ramified) protrusions to carry out their sentinel function in the central nervous system (CNS), which is responsible for monitoring changes in the neuronal microenvironment. Activation of microglia leads to a loss of ramification, enabling them to take on an ameboid cell morphology. This morphological change enhances their motility and capacity for phagocytic activities.
In Alzheimer’s disease, the chronic activation of microglia by neurotoxic proteins — amyloid beta plaques and tau protein aggregates — reduces the ability of microglia to conduct phagocytosis. This, in turn, exacerbates amyloid accumulation, tau propagation, and neuronal death, ultimately promoting disease progression. Indeed, the ramified population of microglia is decreased in Alzheimer’s disease patients. Therefore, monitoring the change in the ramified surveillant state of microglia to their ameboid state is critical for predicting and potentially modifying disease outcomes in neurodegenerative diseases.
Until now, it has remained unclear which molecular pathways are responsible for microglial activation in disease contexts. A novel study by Adrian et al. was able to demonstrate that loss of ramification and microglial activation takes places in Alzheimer’s disease models. This process is promoted by microtubule cytoskeletal rearrangements. Moreover, cyclin-dependent kinase 1 (CDK1) plays a key role in regulating these cytoskeletal changes underlying microglial activation. Employing small-molecule inhibitors of CDK1 to modulate tau- and amyloid beta-induced microglial activation represents a promising potential strategy in the treatment of Alzheimer’s disease.
Neurodegenerative disease models highlight morphological changes in microglia
To investigate which pathways are involved in microglial activation in neurodegenerative diseases, the researchers employed a widely used Alzheimer’s disease transgenic TauP301S mouse model. The microglia of transgenic mice—which overexpressed human tau with a TauP301S mutation—demonstrated decreased ramification in the hippocampus as early as the 6 month mark. This effect was even more pronounced after 12 months. Along with further data, the genetic models of neurodegenerative diseases demonstrated that microglial reactivity leads to morphological changes at pathological stages of neurodegeneration.
Dissecting morphological changes using an in vitro model
In collaboration with Genentech (a member of the Roche Group), the researchers from the Department of Neuroscience in South San Francisco examined the underlying molecular mechanisms of microglial activation and loss of ramified structures. Due to the lack of tissue structure, cultured microglia were much less ramified in vitro than in vivo, rendering the detection of differences in ramification more challenging. For that reason, in addition to measuring the overall branching complexity of microglia using the ramification index, differences in the cell size were also monitored. This data proved to be indispensable, as it was observed that when microglia are stimulated, cell size increases as cells retract their ramifications and transform into an amoeboid or spherical shape.
Treating primary microglial culture with pro-inflammatory bacterial lipopolysaccharide caused loss of ramification in microglia and induced larger cell sizes. In the same way, exposing microglia to recombinant tau—StressMarq’s Tau-441 (2N4R) Wild-Type Monomers (catalog# SPR-479) and Tau-441 (2N4R) P301S Mutant Pre-formed Fibrils (Baculovirus/Sf9) (catalog# SPR-471) also influenced cell size and ramification.
Inhibition of tau and amyloid fibril-induced morphological changes using CDK1 inhibitors
Subsequently, the authors posited that the reorganization of the microtubule cytoskeleton was responsible for the morphological changes in microglial activation. Proteomic profiling was used to pinpoint upstream regulators of microtubule remodelling in microglia, leading to the successful identification of CDK1. Indeed, small molecular inhibitors of CDK1 were able to protect against the increased cell size and reduced ramification that followed LPS induction. Similarly, CDK1 inhibitors proved effective in attenuating the cell size and ramification phenotype associated with the introduction of amyloid beta fibrils and StressMarq’s Tau-441 (2N4R) P301S Mutant Pre-formed Fibrils (Baculovirus/Sf9) (catalog# SPR-471).
Discerning the pathways underlying cytoskeletal reorganization during microglial reactivity
The process by which microglia change from their ramified surveillant state to their ameboid state is critical for predicting or modifying disease outcomes in neurodegenerative diseases. Until now, there has been a gap in understanding which molecular mechanisms participate in microglial activation and loss of their ramified structures in the context of neurodegenerative diseases. The study by Adrian et al., uncovered how microglial activation is triggered by reorganization of the microtubule cytoskeleton.
StressMarq’s Tau-441 (2N4R) Wild-Type Monomers (catalog# SPR-479) and Tau-441 (2N4R) P301S Mutant Pre-formed Fibrils (Baculovirus/Sf9) (catalog# SPR-471) were critical in understanding how toxic aggregates in Alzheimer’s disease can activate microglia, thereby inducing loss of ramification. Following the identification of CDK1 as an upstream regulator of microtubule remodelling in microglial activation, the researchers employed targeted inhibitors to rescue tau and amyloid fibril-induced morphological changes in microglia. The data presented in the study presents the use of CDK1 inhibitors to modulate microglial reactivity in the context of neurodegenerative disease treatment.
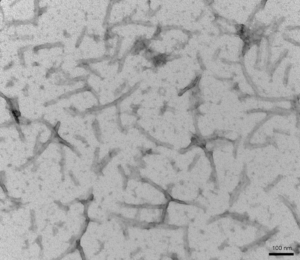
Figure 1. TEM of StressMarq’s Tau-441 (2N4R) P301S Mutant Pre-formed Fibrils (Baculovirus/Sf9) (catalog# SPR-471).
Related StressMarq products
StressMarq manufactures cutting-edge tau, amyloid beta & alpha synuclein protein constructs for neurodegenerative disease research. Visit our website to learn more about our related amyloid beta products, including Amyloid Beta 1-42 Pre-formed Fibrils (catalog# SPR-487) and Amyloid Beta 1-42 Oligomers (catalog# SPR-488), along with the latest scientific publications featuring our diverse product portfolio.
References
- Polarized microtubule remodeling transforms the morphology of reactive microglia and drives cytokine release. Adrian, M. et al. Nature Communications. 2023.
- Microglial morphology in Alzheimer’s disease and after Aβ immunotherapy. Franco-Bocanegra, D. K. et al. Scientific Reports. 2021.


Leave a Reply