FABP2: A Novel Role in Gut Alpha Synuclein Pathology
Synucleinopathies represent a family of neurodegenerative diseases characterized by the abnormal deposition of α-synuclein. Parkinson’s disease (PD) is a well-known synucleinopathy estimated to affect millions of people worldwide. In the context of this progressively debilitating disease, alpha synuclein is converted from its physiologically healthy monomeric form into β-sheet-rich oligomeric and fibrillar forms that readily aggregate into insoluble Lewy bodies (LBs). Furthermore, these toxic alpha synuclein aggregates can propagate within cells, spread to surrounding cells and circulate via the bloodstream.
Recently, new evidence has demonstrated that alpha synuclein is able to propagate from the gut via the vagus nerve before beginning to accumulate in the brain. In Parkinson’s disease, the vagus nerve is posited to be one of the first sites of the central nervous system (CNS) involved in the progression of the disease. Moreover, the relationship between Parkinson’s disease and the gut is not solely relevant at a molecular level but also clinically significant, with patients suffering from the disease reporting gastrointestinal dysfunction in early disease stages and even prior to diagnosis and the development of motor symptoms.
Intestinal FABP2 facilitates alpha synuclein uptake
Until now, little has been known about which protein complexes are involved in alpha synuclein uptake and accumulation in enteric neurons. Research from Sekimori et al. elucidates the role of the intestinal fatty acid-binding protein (FABP2) in regulating the alpha synuclein localization in enteric neurons. The findings revealed that alpha synuclein was taken up in enteric neurons and transported to regions dense in FABP2.
Indeed, monitoring the FABP2/alpha synuclein ratio in plasma samples of patients with Parkinson’s disease allowed more accurate differentiation between disease groups compared to healthy control patients than detecting alpha synuclein alone. The FABP2/alpha synuclein ratio can potentially be used as a plasma biomarker for monitoring Parkinson’s disease progression, making it of invaluable significance to researchers seeking to understand PD pathology on a deeper level.
FABP2 promotes alpha synuclein accumulation in enteric neurons
To begin the investigation, researchers from Tohoku University in Japan defined a primary culture model of murine enteric neurons. In this model, it was important to study the interplay between alpha synuclein and transport lipids, such as fatty-acid binding proteins (FABPs). Different subtypes of FABPs have different localization sites; in the brain, FABP3 has already been defined to play an important role in the intracellular uptake of alpha synuclein and the pathogenesis of synucleopathies. FABP2 is the predominant subtype of interest in the intestine. Commercially available alpha synuclein pre-formed fibrils (PFFs) from StressMarq have been shown to induce Lewy body pathology by providing seeds for the aggregation of endogenous alpha synuclein
To observe the effect of FABP2 on disease pathology in this enteric neuronal model, StressMarq’s fluorescently tagged Alpha Synuclein Pre-formed Fibrils: ATTO 594 (catalog #SPR-322-A594) were added to the neuronal medium. Uptake and retention of the alpha synuclein PFFs over time were monitored in a non-invasive manner. Post-fixation, immunocytochemistry and confocal microscopic analysis were performed and involved monitoring the fluorescent alpha synuclein PFFs along with FABP2 and the neuronal marker, βIII-Tubulin. The collected data demonstrated that alpha synuclein accumulation was significantly higher in areas where FABP2 was more concentrated when compared to areas where FABP2 was absent. Furthermore, a positive correlation was observed between fluorescence intensity measurements of alpha synuclein and FABP2 staining.
Employing the FABP2/alpha synuclein ratio as a plasma biomarker
Monitoring Parkinson’s disease progression is critical in evaluating the efficacy of treatments seeking to slow disease progression. The researchers continued their investigation by assessing the measurement of alpha synuclein and FABP2 in a clinical context. FABP2 plasma levels initially increased with increasing disease progression, peaked at 5–10 years after disease onset and then decreased. Alpha synuclein also displayed consistent fluctuation patterns over the duration of the disease.
In patients with Parkinson’s disease, plasma alpha synuclein initially peaked approximately 5 years after the onset of the disease, decreased once and then increased once more. Due to the complex movement of the accumulated toxic forms of alpha synuclein into other cells, tissues and the bloodstream, plasma levels of total alpha synuclein did not necessarily rise in response to disease progression.
At this point in the study, the researchers defined a new index — the FABP2/alpha synuclein ratio — by measuring both proteins in plasma for the duration of the disease. The new index improved the discrimination of patients with Parkinson’s disease 2–5 years post-onset and control patients, compared with monitoring FABP2 or alpha synuclein alone. It was concluded that the strong discriminatory ability of the index made it a robust candidate as a plasma biomarker for Parkinson’s disease.
Potential inhibition of the FABP2/alpha synuclein complex
To conclude the study, the researchers discussed the broader context of their research findings. Excitingly, their findings thus far had already investigated alpha synuclein protein partners and found that FABP3 in the brain is essential for the intracellular uptake and propagation of alpha synuclein. By developing a selective ligand for FABP3, inhibition of the FABP3-alpha synuclein protein complex resulted in an impediment of alpha synuclein oligomerization and spreading in the brain.
These recent findings provided the first evidence of the contribution of FABP2 in the pathogenesis and progression of alpha synuclein in the intestine, which could play a key role in the initial stages of Parkinson’s disease before propagation from the enteric nervous system to the vagus nerve and CNS. One possible product of the research into FABP2 and alpha synuclein could be the development of ligands to inhibit the FABP2-alpha synuclein protein complex formation and the consequent unpredictable oligomerization process that contributes to the progression of synucleinopathies.
Elucidating the role of FABP2 in enteric neurons utilizing StressMarq’s alpha synuclein constructs
In this study, StressMarq’s fluorescently tagged Alpha Synuclein Pre-formed Fibrils: ATTO 594 (catalog #SPR-322-A594) were crucial in defining how FABP2 contributes to the pathogenesis and progression of synucleinopathies such as Parkinson’s disease. Moreover, employing the FABP2/alpha synuclein ratio improved the discrimination of plasma samples between those belonging to patients with Parkinson’s disease and those belonging to healthy patients.
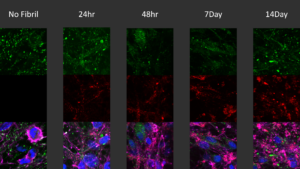
[Image retrieved from StressMarq Biosciences.] Immunocytochemistry/immunofluorescence analysis of human iPSC-derived neurons treated with ATTO 594-labelled Alpha Synuclein Pre-formed Fibrils (catalog #SPR-322-A594). Green: Anti-Alpha Synuclein (pSer129) Antibody (catalog #SMC-600) 1:5000; Red: ATTO 594-labelled Alpha Synuclein Pre-formed Fibrils (catalog #SPR-322-A594). Pink: actin; Blue: Hoechst / DNA.
Conclusions
Patients with Parkinson’s disease often experience gastrointestinal issues in the early stages of the disease, before motor symptoms present themselves. FABP3 has previously been shown to promote alpha synuclein fibrillization and consequent neurotoxicity in the brain. The intestine expresses another subtype of FABPs — FABP2, which, in the study by Sekimori et al. is shown to potentially contribute to the pathogenesis and progression of synucleinopathies by aiding the accumulation of alpha synuclein in enteric cells.
Measuring the FABP2/alpha synuclein ratio allows for more accurate discrimination in plasma samples between patients suffering from Parkinson’s disease and healthy patients, compared to detecting alpha synuclein alone. The data presented in this study support the use of FABP2 as a biomarker for monitoring disease progression of Parkinson’s disease as well as defining the molecular interactions occurring before the propagation of alpha synuclein from the enteric nervous system to the CNS.
Related StressMarq products
StressMarq manufactures a wide range of cutting-edge alpha synuclein protein constructs for neurodegenerative disease research. Related tools for studying cell entry and localization include the Alpha Synuclein E114C Mutant Pre-formed Fibrils: ATTO 488 (catalog #SPR-518-A488) & Monomers (catalog #SPR-517-A488). Visit our website for more information, including the latest scientific publications using our specialized alpha synuclein, tau & amyloid beta protein constructs.
References
- FABP2 is involved in intestinal α-synuclein pathologies. Sekimori, T., et al. J Integr Neurosci. 2024.
- Stages in the development of Parkinson’s disease-related pathology. Braak H et al. Cell and Tissue Research. 2004.
- Gastrointestinal dysfunction in Parkinson’s disease. Pfeiffer RF. The Lancet Neurology. 2003.


Leave a Reply