A Neuron/Astrocyte Model of Tau Pathology
The nervous system consists of two types of essential entities: neuronal cells and glial cells. Astrocytic glial cells, more commonly known as astrocytes, comprise most of the glial cells in the central nervous system (CNS). This sub-type has long been regarded as a passive provider of neurotrophic support to neurons – one of many important maintenance roles that astrocytes assume in the brain. In recent years, a growing body of evidence has demonstrated that astrocytes actively control neuronal functions and communication pathways and independently regulate neuroactive substances. Furthermore, they have been shown to shed their homeostatic roles and adopt a more neurotoxic function within the context of neurodegenerative diseases such as Alzheimer’s and Parkinson’s.
Generated for the first time in 2007, human-induced pluripotent stem cells (hiPSCs) represent a valuable model for understanding neurodegenerative diseases. These stem cells are able to propagate indefinitely, and can differentiate into various cell types, including neuronal cells. Yet, to date there has been a lack of effective, reproducible human co-culture models that include human neurons and astrocytes, consequently contributing to a limited understanding of the influence of astrocytes on intraneuronal tau aggregation.
The comprehensive protocols published by Batenburg et al. in Current Protocols details the establishment of hiPSC and human primary astrocyte co-cultures. hiPSCs — differentiated into pathogenic cell types, alongside lentiviral overexpression of mutated tau variants and seeding with mutant pathogenic tau fibrils — model Alzheimer’s disease pathophysiology. This human co-culture model represents an indispensable tool for identifying novel drug targets and developing therapeutic interventions for human tauopathies.
A human neuron/astrocyte co-culture model
Developing an effective, reproducible hiPSC-derived co-culture model is a complex and challenging undertaking. Researchers must contend with issues such as variability of neuronal differentiation, cell clustering, and unpredictable culture detachment. Inefficient tau aggregation and difficulty successfully establishing a co-culture using astrocytes has also posed significant hurdles. Researchers from Vrije Universiteit in Amsterdam successfully overcame these challenges to design a robust co-culture model of hiPSC-derived neurons and primary astrocytes in a 96-well format compatible with high-content microscopy. With its meticulous design and diverse testing capabilities, this model may offer a reliable and reproducible platform for studying tau pathologies.
Scientists employed hiPSCs with a stably integrated, doxycycline-inducible neurogenin 2 (Ngn2) transgene. Expression of transcription factor neurogenin 2 (NGN2) has been known to induce the differentiation of hiPSCs into functional neurons. The differentiation process is commonly coupled with brightfield imaging microscopy. Prior to differentiation, hiPSCs appeared as tight colonies with smooth edges. Following induction by Ngn2, neurite-like protrusions could be observed. In addition, co-localization of presynaptic synaptophysin 1 (SYP1) and postsynaptic density protein 95 (PSD95) puncta by immunostaining and confocal microscopy revealed that the neurons in the co-cultures formed morphologically mature synapse patterns that were indicative of mature neuronal cells.
Geltrex treatment was also utilized to prevent cell clustering and culture detachment, allowing the neuron/astrocyte co-culture model to avoid a common limitation observed in other culture models. Furthermore, the addition of Geltrex yielded superior culture integrity in comparison with cultures treated with poly-L-ornithine and laminin, and allowed the neurons to form dense healthy networks.
Transduction of neural progenitors using mutated tau variants
Following differentiation, lentiviral transduction was performed with tau variants linked with frontotemporal dementia. The P301L mutant — where the proline is replaced by leucine at amino acid 301 — is a prevalent pathogenic mutation commonly associated with frontotemporal dementia. Moreover, it exhibits accelerated tau pathology and cognitive impairments in cellular and mouse models.
In Alzheimer’s disease and other dementias, tau becomes neurotoxic by forming aggregates that progressively accumulate and lead to neuronal dysfunction and death. Disease progression is further aided by the ability of misfolded tau aggregates to recruit endogenous tau in a “prion-like” mechanism called seeding. Lentiviral overexpression of tau containing the P301L mutation in the hiPSCs-derived neurons did not result in intraneuronal tau aggregates. Therefore, tau fibrils containing the same mutation as the variant of interest – StressMarq’s Tau (K18) P301L Mutant Pre-formed Fibrils (catalog# SPR-330) – were required to stimulate the aggregation of monomeric tau.
Tau pathology in neuron/astrocyte co-culture model
Analysis of tau pathology was undertaken using conventional co-culture cell fixation methods. This process included immunostaining with the MC1 antibody, which recognises conformationally altered and aggregated tau. In brain tissues, increased MC1 reactivity correlates to the severity and progression of Alzheimer’s disease, with no cross-reactivity shown against control brain samples.
Methanol fixation of the co-culture model removed soluble tau and permitted the analysis of only insoluble aggregated tau fibrils. Immunostaining with the AT100 antibody, which recognizes a unique double-site phospho-epitope AT100 (Thr212/Ser214) on hyperphosphorylated tau, revealed positive reactivity with the neuron/astrocyte co-culture model expressing the P301L mutant seeded with mutant PFFs. Taken together, the data from the co-culture model expressing the P301L mutant and seeded with StressMarq’s P301L mutant tau PFFs were indicative of increased deposition of misfolded and aggregated tau. The results were conclusive for the formation of abnormally hyperphosphorylated tau — the hallmark molecular change associated with Alzheimer’s disease.
Induction of seeding using StressMarq’s tau PFFs
Neurodegenerative disease models have previously been limited by cells derived from non-human sources or technical challenges that have hindered the generation and reproducibility of a suitable neurodegenerative culture model. The findings from Batenburg et al. describe a neuron/astrocyte co-culture model able to successfully reproduce Alzheimer’s disease mechanisms. The model was able to overcome cell clustering and culture detachment challenges and enable the formation of a dense neuronal network in hiPSC-derived neuronal culture. Robust seeding was induced using StressMarq’s Tau (K18) P301L Mutant PFFs (catalog# SPR-330), in order to effectively model the molecular mechanisms giving rise to cognitive impairments observed in neurodegenerative disease progression.
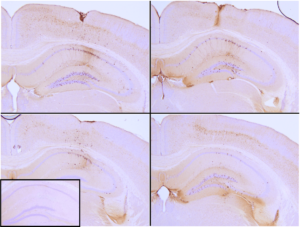
[Image from: StressMarq website.] Immunohistochemistry analysis of P301L mouse hippocampus injected with StressMarq’s Tau (K18) P301L Mutant Protein Pre-formed Fibrils (catalog# SPR-330). Seeding of tau pathology observed at injection site nine weeks post-injection. Inset: negative control. Experiments performed at reMYND N.V.
Summary
Pre-clinical screening of tau-targeting therapies has traditionally focused on neuronal cultures, but the crucial role of astrocytes in supporting neuronal function is increasingly being recognized. The recent findings published by Batenburg et al., describe a neuron/astrocyte co-culture model compatible with multiple microscopy techniques. Lentiviral overexpression of the P301L mutant tau variant in a neuron/astrocyte culture coupled with StressMarq’s Tau (K18) P301L Mutant PFFs (catalog# SPR-330) was able to induce the formation of insoluble, phosphorylated and pathological tau aggregates. The data presented depicts a cost-effective neuron/astrocyte co-culture model that can express tau variants with inducible tau seeding. This model provides a more accurate representation of the complex environment in the brain, and has the potential to improve and accelerate drug development research for tauopathies.
Related StressMarq products
StressMarq manufactures a wide range of cutting-edge tau protein constructs for neurodegenerative disease research. Related tools for studying the aggregation of tau variants include the Tau-441 (2N4R) P301S Mutant: ATTO 488 (catalog# SPR-329-A488) & Tau (K18) Delta K280 Mutant (catalog# SPR-477) Pre-formed Fibrils. Visit our website for more information, including the latest scientific publications using our specialized alpha synuclein, tau & amyloid beta protein constructs.
References
- hiPSCs for predictive modelling of neurodegenerative diseases: dreaming the possible. Rivetti di Val Cervo, P. et al. Nat Rev Neurol. 2021.
- A human neuron/astrocyte co-culture to model seeded and spontaneous intraneuronal tau aggregation. Batenburg, K. L. et al. Curr Protoc. 2023.
- Good, bad, and neglectful: Astrocyte changes in neurodegenerative disease. Jiwajia, Z. et al. Free Radic Biol Med. 2022.
- Intraneuronal tau aggregation induces the integrated stress response in astrocytes. Batenburg, K. L. et al. J Mol Cell Biol. 2022.

Leave a Reply