Early Detection of Alzheimer’s Disease Using Imaging Probes
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder estimated to affect millions of individuals worldwide. The predominant symptoms such as memory loss, cognitive decline, and behavioural changes greatly impact quality of life in afflicted individuals, while simultaneously presenting a significant challenge for families, caregivers, and the healthcare system. At the molecular level, this incurable disease is thought to be caused by the abnormal accumulation and misfolding of the amyloid beta peptide in the brain.
Toxic amyloid beta is generated by protease cleavage of the amyloid precursor protein (APP), and aggregates into oligomers, protofibrils, fibrils, and ultimately, plaques. This pathological process is observed in several neurodegenerative diseases, including Alzheimer’s disease. Initially proposed by Hardy and Higgins (1992), the amyloid cascade hypothesis postulates that the formation of insoluble amyloid beta aggregates represents the central event in the pathogenesis of Alzheimer’s disease. However, a vast body of scientific evidence from the last three decades has indicated that the soluble forms of amyloid beta, such as oligomers, are the primary entity driving neurotoxicity, disease progression, and severity of AD symptoms. Therefore, the detection of soluble amyloid beta is crucial for the accurate diagnosis of early-stage Alzheimer’s disease.
Near-infrared fluorescence (NIRF) imaging using compatible probes represents a promising technique for in vivo imaging of amyloid beta in the brain. It is especially advantageous due to its high level of chemical stability and minimal cytotoxic effects. Until now, identifying a NIRF probe that specifically binds to soluble amyloid without any cross-reactivity with insoluble species has proven to be a difficult undertaking. The findings by Akasaka et al. published in the Journal of Medicinal Chemistry, have overcome this challenge by designing and optimizing a synthetic BODIPY derivation, BAOP-16, to specifically bind to the early phase marker of AD — soluble amyloid beta. This non-invasive technique could potentially optimize NIRF imaging for the sensitive and accurate detection of early markers of Alzheimer’s disease.
Identifying a fluorescent imaging probe
The first steps of the study involved identifying a NIRF-compatible fluorescence probe, which, upon binding to the target, produces a fluorescent signal in the near-infrared (NIR) region (650 nm-900 nm). The researchers from the Patho-Functional Bioanalysis group at Kyoto University had previously developed boron dipyrromethene BODIPY NIR fluorescent (NIRF) probes targeting amyloid beta structures. The initial investigation began by testing whether the previous chemical designs also bound to soluble amyloid beta aggregates. At this stage, it was important to comprehensively classify the amyloid structures and ensure that the designed NIRF probes targeted soluble forms of amyloid beta.
Next, structural analysis using transmission electron microscopy (TEM) was used to categorize the conformational structures of amyloid beta. The transition from soluble amyloid beta protein species to insoluble aggregates is linked to a distinct structural alteration. In this process, the soluble species undergo a conformational change to form cross-β-sheet fibrils, where the β-strands are oriented perpendicular to the fibril axis. This transformation is conducive to creating stable, densely packed plaques in the brain tissue. Finally, TEM analysis revealed that the soluble aggregate forms appeared spherical, while the insoluble forms were fibrillar in nature.
In brief, the initial results indicated that the BODIPY structure was a suitable scaffold, and the NIRF probes produced a strong fluorescence intensity when binding to amyloid beta soluble aggregates rather than amyloid beta fibrils. Once the chemical scaffold was chosen, the scientists proceeded to optimize reaction conditions by modifying the conjugation systems and functional groups, with an emphasis on improving the structure’s specificity. Subsequently, structural-functional relationship studies assessed the fluorescence properties of the dyes — including the absorption, excitation, emission wavelengths and quantum yield — and their ability to specifically bind soluble amyloid beta aggregates. Following a series of chemical synthesis experiments, a fluorescence probe named ‘BAOP-16’ was chosen. Interestingly, the NIRF BAOP-16 probe had a unique branched chemical structure, which resembled a “y” shape and was designed to enable steric hindrance and improve binding to amyloid beta oligomers.
Selective binding of soluble amyloid beta
One key criterion for the chosen NIRF probe was to ability to selectively bind soluble amyloid beta aggregates. To achieve this, different species of amyloid beta were used to assess the targeting behaviour of BAOP-16. Two types of soluble aggregate forms were assessed: amyloid beta oligomers and amyloid beta protofibrils, with the former being the soluble precursor to the insoluble fibrillar forms. Binding affinity studies confirmed that the binding to soluble forms of amyloid beta was in the high-affinity range: binding to the oligomers yielded a dissociation constant (Kd) value of 13.9 nM while the protofibrils garnered a Kd of 15.1 nM. Furthermore, binding to insoluble amyloid beta fibrils and monomers was not observed.
Protein aggregation is a common hallmark event across various neurodegenerative diseases. Another crucial experimental consideration was excluding the non-specific binding of the NIRF probe to other neurodegenerative protein aggregates. One such protein, tau, is known to abnormally aggregate and generate neurofibrillary tangles in neurodegenerative disease pathology. No binding was seen when the BAOP-16 fluorophore was introduced to StressMarq’s Tau (K18) Delta Mutant PFFs (catalog# SPR-477), increasing confidence in the specific nature of the NIRF BAOP-16 probe.
In vitro and in vivo fluorescence staining
To ascertain the potential use of the NIRF probe in humans, measuring its uptake in the brains of animal models was an important experimental parameter. Subsequently, the NIRF probe was tested to determine whether it could be used to detect soluble amyloid beta aggregates in mouse brains in vivo. Here, the mice were treated with soluble amyloid beta, which demonstrated reactivity with the BAOP-16 probe. Thereafter, another Alzheimer’s disease mouse model was employed to further develop confidence in the specific binding capabilities of the novel probe.
The APPNL‑G‑F/NL‑G‑F knock-in model mouse harbours mutations in the APP gene, resulting in elevated amyloid beta aggregation through increased soluble amyloid beta protein expression, and replication of multiple critical aspects of the disease, including cognitive decline. A lack of extraneous complications arising from the induced APP overexpression made the implementation of this particular transgenic mouse model highly advantageous. Ultimately, the experimental data suggested that the NIRF BAOP-16 probe possessed the ability to accurately detect soluble amyloid beta aggregates derived from the Alzheimer’s disease model.
Identifying NIRF-compatible probes using StressMarq’s PFFs
Over the last three decades, there has been a profound shift in understanding the role of amyloid beta oligomers as principal drivers of neurotoxicity in neurodegenerative diseases. This is in constrast with the previous belief that insoluble plaques were key drivers of disease pathology. Detecting the formation of these soluble aggregate forms of amyloid fibrils in early-stage Alzheimer’s disease using NIRF probes, such as those identified in the study by Akasaka et al., represents a crucial tool in accurately diagnosing patients. StressMarq’s Tau (K18) Delta Mutant PFFs (catalog# SPR-477) were pivotal in ensuring that the selected NIRF probe only targeted soluble forms of amyloid beta, without binding to the other crucial neurodegenerative protein aggregates that also accumulate in Alzheimer’s disease and other forms of dementia.
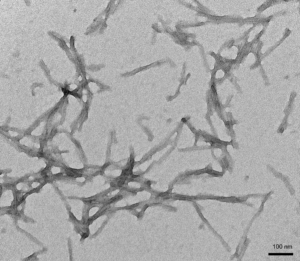
[Image from: StressMarq website] TEM of StressMarq’s Human Recombinant Tau (K18) Delta K280 Mutant Pre-formed Fibrils (catalog# SPR-477).
Summary
There is mounting evidence supporting the role of soluble amyloid beta aggregates as a central player in mediating neurotoxicity in Alzheimer’s disease. Designing near-infrared fluorescence imaging probes for Alzheimer’s disease has been limited by the lack of selective probes able to exclusively target these aggregates. The recent findings by Akasaka et al. have overcome these experimental challenges by designing a NIRF imaging probe containing a BODIPY scaffold that selectively binds soluble amyloid beta with high affinity. The inability to bind StressMarq’s Tau (K18) Delta Mutant PFFs (catalog# SPR-477) demonstrated that the NIRF probe exclusively bound the desired target without binding to other neurodegenerative protein targets. Currently, early detection of Alzheimer’s disease is made difficult due to symptom prominence increasing with disease progression. Imaging with NIRF probes targeting early neurotoxic soluble forms of amyloid beta could increase the accurate early diagnosis of Alzheimer’s disease, opening up more treatment and support options for patients, families and health care professionals.
Related StressMarq products
StressMarq manufactures a wide range of cutting-edge tau protein constructs for neurodegenerative disease research. Visit our website for more information, including the latest scientific publications using our specialized tau, alpha synuclein & amyloid beta protein constructs.
References
- In vivo near-infrared fluorescence imaging selective for soluble amyloid β aggregates using y-shaped BODIPY derivative. Akasaka, T et al., J Med. Chem. 2021.
- Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Mucke, L et al., Cold Spring Harb. Perspect. Med. 2012.
- Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Ferreira S.T. et al., Cell. Neurosci. 2015.

Leave a Reply