Regulating the Pathological Transmission of Alpha Synuclein
Parkinson’s disease (PD) is a neurodegenerative disease in which the cells in the brain that regulate motor function progressively deteriorate. Its prevalence is estimated to have doubled in the past 25 years, with recent estimates suggesting that over 8.5 million individuals are living with Parkinson’s disease today. Those afflicted with PD display both motor and non-motor symptoms. The cardinal motor symptoms include loss of speech, slowness of movement (bradykinesia), and changes in normal walking patterns.
It has been established through years of research efforts that the driving force behind the development of these motor symptoms is the accumulation of misfolded alpha synuclein into insoluble Lewy bodies. This process results in the disruption of neuronal cellular function, and promotes dopamine deficiencies. As dopamine is a key neurotransmitter for the regulation of movement, this progressive loss of dopaminergic neurons leads to the emergence of the devastating motor symptoms observed in patients with Parkinson’s disease. Cell-to-cell transmission of alpha synuclein aggregates is vital to the spread of disease pathology between neurons and astrocytes in different brain regions.
A recent study by Luo et al. published in Neural Regeneration Research revealed that the transmembrane protein involved in endocytosis — lipoprotein receptor-related protein 1 (LRP1) — plays a critical role in the spread of pathological alpha synuclein across different regions of the brain. Animal PD models provided compelling evidence that the LRP1 receptor regulates cellular transmission between regions in the nigrostriatal system — critical for controlling movement — by mediating alpha synuclein internalization. These recent insights into the regulatory role of LRP1 in alpha synuclein internalization and transmission may be extremely valuable for scientists and clinicians seeking to develop therapeutic interventions for both Parkinson’s disease and other synucleinopathies.
LRP1 and neurodegeneration
At the time of the study’s initiation, links between the endocytic receptor, LRP1, and various neurodegenerative diseases had already been established in published scientific literature. Elevated LRP1 protein levels have been reported in patients with Parkinson’s disease, and furthermore, LRP1 has been shown to regulate tau – a protein whose misfolding drives the pathogenesis of Alzheimer’s disease. The relationship between LRP1 and neurodegeneration has been additionally substantiated by the discovery that the LRP1 adaptor protein is the strongest genetic risk factor for multifactorial forms of late-stage onset Alzheimer’s disease. Despite established data on the role of LRP1 in neurodegneration, researchers identified a lack of information on the connection between LRP1 and the mechanisms influencing alpha synuclein uptake and propagation. The study described herein was subsequently designed and conducted to evaluate the precise role of LRP1 in alpha synuclein pathology.
Recapitulating Parkinson’s disease pathology
Neurodegeneration is a direct consequence of the misfolding and aggregation of alpha synuclein monomers into oligomeric structures, which rapidly accumulate into insoluble pre-formed fibrils (PFFs) in the brain. In order to recreate the pathological hallmarks of Parkinson’s disease in vivo, animal models were induced through the introduction of StressMarq’s Alpha Synuclein Pre-formed Fibrils (catalog #SPR-322) to the striatum region of the brain of cynomolgus monkeys and C57BL/6 mice.
Parkinson’s disease symptoms, including bradykinesia, were successfully observed post-injection. This was accompanied by a significant loss of dopaminergic neurons in the striatal (STR) region, and an increase in LRP1 levels. Interestingly, this loss of dopaminergic neurons was also seen in the substantia nigra (SN) region, suggesting the active transmission of disease pathology from one region of the brain to another.
Next, it was essential to determine whether the increased LRP1 levels seen in patients with Parkinson’s disease were also observed in the animal models. Indeed, a marked increase of LRP1 protein was evidenced in both the STR and the SN brain regions in both the mouse and the monkey models. The data revealed conclusively that alpha synuclein pathology induced by alpha synuclein fibrils could be transported from the STR to the SN region, and LRP1 was responsible for mediating this process. Both of these regions are crucial parts of the nigrostriatal system, where the STR processes dopaminergic input to regulate motor control and the SN produces dopamine.
Protective role of LRP1 downregulation
Although valuable information regarding the pathogenic function of fibrillar alpha synuclein was steadily emerging as the study progressed, the molecular mechanisms underlying Parkinson’s disease initiation and progression remained unclear. Deciphering the nature of the interaction between alpha synuclein and LRP1 was the crucial next step in understanding alpha synuclein regulation. To probe the alpha synuclein and LRP1 relationship further, the PC12 neuronal cellular model was employed. Following incubation of the PC12 cells with different concentrations of StressMarq’s Alpha Synuclein Pre-formed Fibrils (catalog #SPR-322), cells were assessed for viability. Treatment with aggregated fibrillar alpha synuclein exerted a neurotoxic effect and promoted a dose-dependent reduction in cell viability.
Subsequent Western blot analysis of the cell lysates demonstrated increased alpha synuclein and LPR1 levels. Finally, visualization of the neuronal cells via immunofluorescence and confocal microscopy confirmed the uptake of exogenous fibrillar alpha synuclein and quantified a 220% increase in LRP1 signal intensity. However, the addition of StressMarq’s Alpha Synuclein Monomers (catalog #SPR-321) did not induce the same significant increase in LPR1 signal upon addition to the PC12 model.
In the final phase of the study, the researchers posited whether modulating LPR1 protein levels could also regulate the pathological build-up of alpha synuclein. To assess this parameter, the knockdown of LRP1 efficiently inhibited the increase of alpha synuclein in the nigrostriatal system, successfully rescuing neurodegeneration. Furthermore, blocking the lysine residues of alpha synuclein PFFs decreased the concomitant protein increases seen in cultured neuronal cells. It was therefore concluded that LRP1 regulates uptake and transmission by interacting with the lysines at the N-terminus of alpha synuclein.
Regulation of alpha synuclein transmission
In this study, researchers uncovered the role of the LRP1 endocytic receptor in the transmission of pathogenic alpha synuclein throughout regions of the nigrostriatal system. Induction of Parkison’s disease pathology in vivo and in vitro, using StressMarq’s Alpha Synuclein Pre-formed Fibrils (catalog #SPR-322) and Monomers (catalog #SPR-321), enabled researchers to study the regulation of alpha synuclein internalization and subsequent transmission to different regions of the brain.
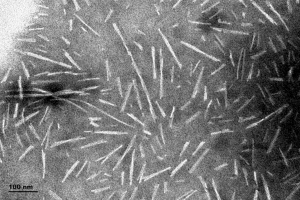
[Image from: StressMarq website] TEM of StressMarq’s Human Alpha Synuclein Pre-formed Fibrils (catalog #SPR-322).
Summary
Alpha synuclein transmission between different regions in the brain actively enables the spreading of toxic insoluble aggregates that are associated with the onset and worsening of motor and cognitive symptoms in Parkison’s disease. Although strong links have been shown to exist between alpha synuclein and the endocytic receptor, LRP1, the molecular mechanisms underlying this relationship have previously remained unknown. The recent findings by Luo et al. describe the regulatory role of LRP1 in alpha synuclein uptake and propagation through the use of animal in vivo models and cellular in vitro models. Alpha synuclein and LRP1 levels are elevated in PD models, and the pathological accumulation of alpha synuclein can be reversed by masking N-terminal lysine residues in fibrillar alpha synuclein aggregates. The results reported herein constitute a possible step forward in developing strategies to prevent Parkinson’s disease, and the advancement of neurodegenerative disease research in its entirety.
Related StressMarq products
StressMarq’s diverse range of alpha synuclein protein constructs enables researchers to create high-quality neurodegenerative disease models. This extensive portfolio includes Alpha Synuclein N-Terminal Acetylated PFFs (catalog# SPR-332) and Monomers (catalog# SPR-331). Visit our website for more information, including the latest scientific publications using our specialized alpha synuclein, tau and amyloid beta proteins for neurodegenerative disease research.


Leave a Reply