Reversing Tau Pathology Through Mitochondrial Regulation
It has been well established through decades of scientific discovery that mitochondria are the eukaryotic organelles responsible for producing the chemical energy required to power biochemical reactions within the cell. In addition to energy production, mitochondria play essential roles in calcium buffering, apoptosis, and inflammation. Due to their involvement in these diverse cellular processes, mitochondrial dysfunction is implicated in several age-related diseases, including neurodegenerative diseases.
In one of the most common neurodegenerative diseases, Alzheimer’s disease (AD), mitochondrial dysfunction has been observed alongside the onset of disease symptoms. It is thought that the relationship between mitochondrial dysfunction and AD is primarily driven by the toxic accumulation of the hyperphosphorylated tau protein and subsequent formation of neurofibrillary tangles (NFTs) in the brain, as well as the induction of oxidative stress.
Regulation of mitochondrial activity
Adiponectin receptors are essential regulators of mitochondrial function. These receptors — of which there are two isoforms, AdipoR1 and AdipoR2 — mediate the signalling of the hormone adiponectin, a fat-derived hormone that plays a critical role in the maintenance of metabolic homeostasis. Metabolic abnormalities including obesity, insulin resistance, type 2 diabetes, and cardiovascular disease are associated with decreased adiponectin levels. Exertion of adiponectin functions through these transmembrane receptors enables the control of glucose uptake and lipid metabolism. Furthermore, regulating adiponectin receptor activity is achievable by using selective adiponectin receptor agonists that bind and activate AdipoR1 and AdipoR2 to mediate metabolic activity.
Although the link between mitochondrial dysfunction and Alzheimer’s disease has been established, the precise mechanisms driving this relationship remain relatively unknown. The findings from McGregor et al. investigated whether mitochondrial targeting using adiponectin receptor agonists could be beneficial in the context of neurodegenerative disease therapy. Treatment of neuronal tau pathology with the small, synthetic adiponectin receptor agonist AdipoRon demonstrated the ability to reverse mitochondrial and functional electrophysiological defects induced by tau pathology. Moreover, the researchers elucidated the molecular pathways underlying the reversal of neuronal tau pathology. This research effectively outlined the molecular pathways linking adiponectin receptor activation with tau pathology and may contribute to the identification of novel therapeutic targets for the treatment of Alzheimer’s disease.
Reversal of neuronal tau pathology
As described in the paper, the researchers from the University of Wisconsin-Madison, USA selected primary neurons derived from a commonly employed mouse model as the scaffold for their study on the role of adiponectin receptors in neurodegenerative diseases. The PS19 transgenic mouse model harbours mutations in human microtubule-associated protein tau (MAPT). In this model, the expression of the transgenes encoding the disease-associated P301S mutation was under the control of a mouse prion protein promoter, which augmented protein expression to approximately five times the endogenous level. Inclusion of the P301S mutation increased the rate of protein misfolding and the formation of NFTs, and also induced neuroinflammation and mitochondrial dysfunction.
StressMarq’s Tau-441 (2N4R) P301S Mutant PFFs (catalog# SPR-329) were subsequently utilized to aid in the replication of Alzheimer’s disease pathology. The inclusion of these specialized protein constructs was essential for seeding tau aggregation and promoting mitochondrial dysfunction.
As expected, the seeding effect of the tau fibrils triggered hyperphosphorylation of the tau protein at residues Ser202 and Thr205, further enhancing tau aggregation and mislocalization. As mitochondria began to degenerate, neurons became increasingly vulnerable to apoptosis. Taken together, these processes exacerbated the neurodegenerative activities commonly correlated with Alzheimer’s disease. Ultimately, activation of adiponectin receptors with AdipoRon circumvented this pathogenic series of events and significantly reduced both the phosphorylation of Ser202 and Thr205 and the presence of NFTs.
Tau clearance through molecular pathways
The next phase of the study involved deciphering the molecular mechanisms employed by adiponectin receptors in tau clearance. A well-characterized downstream mediator of adiponectin signalling is the AMP-activated protein kinase (AMPK). Treatment with the AMPK inhibitor, Compound C, impaired the clearance of hyperphosphorylated tau. Further probing into the downstream adiponectin receptor signalling pathway revealed the involvement of the glycogen synthase kinase-3 beta (GSK3b). The latter is an established tau kinase that contributes to tau hyperphosphorylation and the formation of NFTs. In brief, adiponectin receptor activation led to tau clearance in a multi-step molecular process that implicated both AMPK and GSK3b. Most importantly, activation of adiponectin signalling demonstrated that NFT formation was reversible.
Adiponectin receptor activation
Subsequently, the researchers postulated whether autophagy (degradation of abnormal or malfunctioning cellular components by lysosomes) could play a part in adiponectin receptor clearance of tau. As AMPK has previously been established as regulator of autophagy, it seemed appropriate to consider autophagy as a potential mechanism by which NFT levels could be alleviated. It was determined that the activation of adiponectin receptors with AdipoRon induced the formation of autophagosomes in an AMPK-dependent manner. This supported the initial theory that autophagy contributed to NFT and tau clearance.
Finally, the researchers studied the functional defects of tau pathology in the primary neuronal model. An additional contributing factor in the cognitive decline associated with Alzheimer’s disease is decreased dendritic complexity and the disruption of electrophysiological activity. Adiponectin receptor activation was able to counteract both functional defects, maintaining dendritic complexity and correcting disruptions to electrical functions.
Inducing tau pathology with StressMarq’s PFFs
Mitochondrial dysfunction is increasingly being acknowledged as a factor in the development of Alzheimer’s disease. Indeed, impairment of the mitochondria is thought to influence multiple cellular processes such as energy production and cellular degradation. In this manner, dysfunctional mitochondria promote neurodegenerative disease progression. The recent findings from McGregor et al. reveal — for the first time — that the adiponectin receptor agonist AdipoRon mediates tau clearance. StressMarq’s Tau-441 (2N4R) P301S Mutant PFFs (catalog# SPR-329) were critical to the establishment of an accurate neuronal tau pathology model. Treatment with tau fibrils seeded tau hyperphosphorylation and aggregation, and subsequently formed neurofibrillary tangles, as is observed in real-world Alzheimer’s disease pathology.
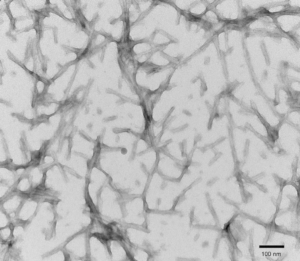
[Image from: StressMarq website] Visualizing fibrillar structures through TEM imaging of StressMarq’s Tau-441 (2N4R) P301S Mutant PFFs (catalog# SPR-329).
Summary
In conclusion, although recent evidence has identified mitochondrial dysfunction as a key contributing factor in Alzheimer’s disease progression, the precise mechanisms behind this pathology have not been described in detail. Providing the first links between adiponectin receptor activation and tau clearance, McGregor et al. defined potential avenues for the mediation of the pathogenic buildup of tau and consequent formation of neurofibrillary tangles.
The neuronal model employed StressMarq’s Tau-441 (2N4R) P301S Mutant PFFs (catalog# SPR-329), which were able to induce key events in Alzheimer’s disease pathophysiology including tau hyperphosphorylation and NFT accumulation. Activating adiponectin receptors using synthetic agonists significantly reduced NFTs and corrected electrophysiological disturbances and dendritic morphological defects, ultimately reversing the established tau pathology. Although they were previously identified as effective regulators of metabolic processes, adiponectin agonists may now be evaluated as potential regulators of tau pathology in neurodegenerative diseases.
Related StressMarq products
StressMarq manufactures a wide range of cutting-edge tau protein constructs for neurodegenerative disease research. This extensive portfolio includes fluorescently-labelled Tau-441 (2N4R) P301S Mutant PFFs: ATTO 488 (catalog# SPR-329-A488) for cellular imaging studies. Visit our website for more information, including the latest scientific publications using our specialized alpha synuclein, tau and amyloid beta proteins for neurodegenerative disease research.
References
- Reversal of neuronal tau pathology via adiponectin receptor activation. McGregor, E.R. et al., Communications Biology. January 2025.


Leave a Reply