Pathogenic Amyloid Fibrils Produced by Gut Microbiota
The increasing prevalence of neurodegenerative diseases in the aging population presents a significant challenge for the modern healthcare system. The hallmark features of these diseases, which include progressive neuronal loss and dysfunction, are primarily induced by the abnormal aggregation of pathogenic amyloid proteins.
Misfolding and self-assembly of soluble amyloids give rise to aggregated insoluble species characterized by a distinctive β-rich fibrillar structure composed of successive layers of β-sheets. These amyloid fibrils can be formed by several different types of proteins and are thought to be implicated in more than 50 human diseases. For example, Parkinson’s disease (PD) is marked by the widespread deterioration of dopaminergic neurons in the substantia nigra, driven by the aggregation of alpha synuclein fibrils into pathological inclusions known as Lewy bodies (LBs).
The formation of insoluble β-sheet-rich alpha synuclein aggregates disrupts cellular processes, activates inflammatory responses, and induces synaptic dysfunction, ultimately facilitating neuronal cell death. Additionally, these aggregated fibrils can propagate between cells by acting as nuclei to initiate alpha synuclein aggregation in recipient cells — a phenomenon known as seeding. This seeding capability enables disease progression and the concurrent worsening of disease symptoms. Proteins of non-human origin can also induce seeding, in a process known as cross-seeding. A significant source of cross-seeding amyloids is the diverse group of bacteria residing in the human gastrointestinal system, collectively known as the gut microbiome.
The gut microbiota biofilm
It has previously been established through scientific literature that the gut microbiota is a significant source of amyloids. In fact, amyloids produced by gut microbiota display the ability to induce a mechanism of autoimmunity known as molecular mimicry, in which host proteins adopt an amyloidogenic β-sheet structure. These bacterial amyloids provide stability to the gut microbiome through the formation of structural scaffolds in the gut known as biofilms. While these biofilm-associated proteins (BAPs) play an essential role in maintaining biofilm integrity, they can also act as virulence factors that facilitate disease pathology.
In the past, characterization of BAP amyloids occurred on a species-by-species basis. However, a need for a more systematic approach has emerged due to the diverse bacterial population in the gut microbiome. Recent research by Fernández-Calvet et al., published in Nature Communications, dissected the amyloidogenic properties of BAPs from the diverse group of bacteria in gastrointestinal biofilms. The study presents direct evidence of the role of BAP amyloids from gut microbiota in promoting alpha synuclein aggregation and neurodegeneration, and opens up new possibilities for early diagnostics and therapeutic interventions.
Molecular signature of biofilm-associated proteins (BAPs)
Biofilms are communities of bacteria, and often proteins, that develop within an extracellular matrix. BAPs with amyloid-like properties have been observed in biofilms formed by enteric bacteria, leading researchers to postulate that these proteins may contribute to the progression of various neurodegenerative diseases. Proteins that undergo a change in conformation from a globular folded state to an amyloidogenic state under certain conditions are known as facultative amyloids. Examples of prototypical facultative amyloids in the gut include Bap from Staphylococcus aureus and Esp from Enterococcus faecalis.
Furthermore, BAPs share a distinct structural homology and are characterized by high molecular weights and a core domain of tandem repeats. Recognizing the widespread presence of BAPs across different bacterial species, scientists from the Institute of Agrobiotechnology in Spain sought to streamline the classification of BAP amyloids and determine whether a shared molecular signature could be identified.
Using BLASTp amino acid sequence analysis, BAP-like genes in the human gut microbiota were identified. Next, shotgun sequencing was utilized to quantify the relative abundance of these species. From 49 patient samples, 11 unique BAP genes from gut microbiota were identified. The data revealed that the BAP genes were part of the accessory genome and were linked to pathogenicity islands, meaning only certain strains of bacteria could produce them. This was consistent with the previously published findings that identified prototypic BAPs from S. aureus and E. faecalis. Interestingly, BAPs from Gram-positive bacteria were less represented within the identified population than those from Gram-negative bacteria.
To determine the clinical significance of these specific BAP genes, the gut microbiome of patients with Parkinson’s disease was analysed alongside that of neurologically healthy individuals. A correlation was observed between the abundance of specific BAP genes in the gut microbiome and Parkinson’s disease incidence.
Insoluble BAP amyloid-like structures
The development of neurodegenerative diseases is closely associated with the aggregation of misfolded proteins into insoluble amyloid fibrils, leading to the formation of toxic neuronal inclusions. These abnormal clumps mediate pathogenesis by ultimately causing neuronal cell loss and dementia. Amyloid fibrils are composed of a cross β-sheet fibrillar structure, which is highly rigid and stable, meaning they remain intact and resistant when subjected to detergents and solvents in the laboratory. Additionally, their dense structures require the combined use of a density gradient and differential centrifugation to generate pellets post-preparation.
To identify whether the amyloid-like structures identified in the gut microbiota also exhibited insoluble properties, the researchers employed these well-defined biochemical protocols alongside well-characterized antibodies. The majority of BAP-positive structures could be detected with StressMarq’s Anti-Amyloid Oligomers (A11) antibody (catalog #SPC-506), which detects oligomeric precursors to amyloid fibrils. Similarly, the same was true when immunoblotting with an amyloid fibril-specific antibody — StressMarq’s Anti-Amyloid Fibril (OC) antibody (catalog #SPC-507). The collected data demonstrated that the BAP structures were found in the insoluble fraction of the human gut microbiota samples and displayed structurally amyloidogenic properties.
Induction of alpha synuclein aggregation
The key molecular factor underlying the progression of neurodegenerative diseases, including Parkinson’s disease, is cell-to-cell transmission. Insoluble, aggregated alpha synuclein fibrils are transmitted by inducing prion-like seeding-based mechanisms that trigger aggregation of soluble native forms of the protein, which can spread within and in between cells. It has been hypothesised that BAP amyloids from the gut may travel to the brain, exacerbating Parkinson’s disease pathology through the induction of cross-seeding.
Using an established in vitro seeding assay, the study then assessed the ability of the BAP-like amyloids from the gut microbiota to induce the formation of intracellular aggregates. For this, BAP-like amyloid fibrils were incubated with StressMarq’s Alpha Synuclein Monomers (catalog #SPR-321), which resulted in the acceleration of alpha synuclein fibrillization. These bacteria-derived amyloids were effectively fibrillized, yielding structures comparable to those of StressMarq’s Alpha Synuclein Pre-formed Fibrils (catalog #SPR-322). Assessment in neuronal models and animal models further corroborated an enhancement in alpha synuclein aggregation, which resulted in dopaminergic neuronal loss and the onset of neurodegenerative symptoms in the respective models.
Investigating amyloidogenic properties of BAP amyloids using StressMarq products
An increasing body of evidence suggests that individuals with neurodegeneration have altered microbiomes, which are correlated with the severity of functional symptoms. Biofilm-associated protein (BAP) amyloids produced by gut microbiota are believed to be a source of amyloids that can travel to the brain, potentially facilitating the progression of Parkinson’s disease through cross-seeding.
The study by Fernández-Calvet et al. characterizes the BAP amyloids from gut microbiota by defining the molecular signature and structural properties of BAPs. Similar to human amyloids, BAP amyloids are found in insoluble fractions as identified by StressMarq’s Anti-Amyloid Oligomers antibody (catalog #SPC-506) and Anti-Amyloid Fibril antibody (catalog #SPC-507). Furthermore, BAPs from gut microbiota displayed pathogenic properties by accelerating the fibrillization of StressMarq’s Alpha Synuclein Monomers (catalog #SPR-321). The BAP amyloids from gut microbiota proved equally as effective as StressMarq’s Alpha Synuclein Pre-formed Fibrils (catalog #SPR-322). Finally, in vitro and in vivo models with BAP amyloids significantly enhanced the development of critical pathological features indicative of Parkinson’s disease.
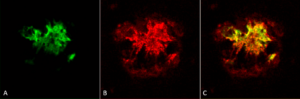
[Image from: StressMarq website] Immunohistochemistry analysis using StressMarq’s (A) Anti-Amyloid Fibrils (OC) Antibody (catalog# SPC-507), (B) Anti-Amyloid Oligomers (A11) Antibody (catalog# SPC-506) and corresponding composite image (C). Courtesy of: Dr. Elizabeth Head, University of California, Irvine.
Summary
Evidence shows that individuals with neurodegenerative conditions have altered microbiomes that correlate with symptom severity. BAP amyloids deriving from gut microbiota represent a significant source of cross-seeding agents that, once transported from the gut to the brain, are able to induce alpha synuclein aggregation. In the past, studies have focused on one bacterial species at a time. However, given the hundreds of diverse bacterial species present in the gut, Fernández-Calvet et al. recognized the need for a novel systematic approach to identifying BAP amyloids.
Taken together, the findings demonstrate that BAPs from gut microbiota are able to form amyloid-like fibrils. Certain BAP genes in the gut microbiome are also correlated with Parkinson’s disease. These BAPs display amyloidogenic characteristics when tested in well-recognized in vitro and in vivo models of alpha synuclein aggregation. Further investigation into BAP amyloids from the gut microbiome may lead to the development of promising tools and strategies for earlier diagnosis and early-stage therapies targeting neurodegenerative pathology.
Related StressMarq products
StressMarq is dedicated to supplying high-quality reagents to support neurodegenerative disease research. We offer a wide range of oligomeric, fibrillar, and monomeric protein preparations and specialized antibodies. These include recombinant monoclonal antibodies targeting amyloid beta 1-42 oligomers. For more information, including the latest scientific publications that utilize our cutting-edge alpha synuclein, amyloid beta, and tau proteins, please visit our website.
References
- Gut microbiota produces biofilm-associated amyloids with potential for neurodegeneration. Fernández-Calvet, A et al. Nature Communications. 2024.

Leave a Reply