Designing Oligomeric Amyloid Beta Quantum Dots
Alzheimer’s disease (AD) is the most prevalent form of dementia, affecting nearly 7 million individuals in the United States – a number that continues to rise rapidly. Research into neurodegenerative diseases presents significant challenges due to the brain’s complexity and the multifactorial nature of disease development. Symptoms and disease progression vary widely, often involving overlapping pathological molecular mechanisms. Neurodegeneration is characterized by several key processes, including protein aggregation, with multiple protein species playing a role.
Amyloid beta is the primary component of extracellular senile plaques in Alzheimer’s disease. Investigating the diverse forms of amyloid beta in neurodegenerative disorders is particularly challenging due to their wide range of conformations, which include monomers, toxic oligomers, fibrils, and plaques. This complexity stems from their dynamic interconversion, structural diversity, varying toxicity levels, and the difficulties associated with isolating and characterizing each species within the brain’s intricate environment.
In recent years, our understanding of the primary triggers of Alzheimer’s disease has evolved. Initially, amyloid beta plaques were thought to be central to neurodegeneration. However, growing evidence suggests that soluble amyloid beta oligomers are the most neurotoxic aggregates. These toxic oligomers first appear as spherical clusters, which later aggregate into protofibrils. They can also activate microglia, leading to inflammation and increased toxicity.
Mimetic quantum dots
Quantum dots are nano-scale particles (2–10 nm in diameter) made of semi-conducting materials. Their fluorescence at specific wavelengths under UV light makes them invaluable for visualizing cellular processes in biological research. Indeed, quantum dots are particularly advantageous due to their brightness, photostability, and long Stokes shift, which collectively improve signal clarity and reduce background noise. These properties make them particularly suited for cell labeling and tracking, such as for neuronal and microglial cells.
Chiang et al. developed quantum dot mimetics for spherical amyloid beta (Aβ) oligomers to replicate their structure while minimizing interference from other protein forms. In doing so, the researchers sought to develop their understanding of the role of Aβ oligomers in neuroinflammation and tau-related pathologies associated with Alzheimer’s disease. Amyloid beta is generated from the cleavage of the amyloid precursor protein (APP). One of the most prevalent forms of amyloid beta, Aβ42, comprises 42 amino acids and is considered to be a major component of AD plaques. The study parameters leveraged the N-terminus of Aβ42, a region crucial for fibrillization, secondary structure formation, and neurotoxicity, in order to create quantum dots with a CdSe/CdS core and shell structure, encapsulated with a DSPE-PEG2k-conjugated Aβ42 peptide.
In order to ensure that the amyloid beta quantum dots were comparable to native oligomeric conformations, structural parameters such as size, shape, and surface characteristics were assessed. These quantum dots were then compared with native spheroidal Aβ oligomers, using StressMarq’s well-characterized Amyloid Beta 1-42 Oligomers (catalog# SPR-488) as a reference. Analytical data confirmed that the quantum dot mimetics closely replicated the morphology of natural Aβ oligomers, offering a valuable tool for understanding neurodegenerative disease processes.
Measuring immunoreactivity
Conventional antibodies were subsequently employed to determine whether the Aβ42 molecules were displayed in the correct oligomeric orientation to mimic neurotoxic, endogenous amyloid beta. Primary rat neuroglial cultures were treated with either amyloid beta quantum dots or native spheroidal amyloid beta oligomers, followed by immunofluorescent labeling to facilitate imaging.
StressMarq’s Anti-Amyloid Oligomers (A11) Antibody (catalog #SPC-506), was selected due to its well-established ability to detect the conformational specificity of amyloid oligomers, which serve as precursors to amyloid fibrils. The conformational structures of soluble oligomers are independent of amino acid sequence, and the A11 antibody recognizes a common peptide backbone epitope exclusive to these soluble oligomers—an epitope absent in monomeric or mature fibrillar forms.
As a result, the A11 antibody is widely recognized for its effectiveness in identifying misfolded amyloid oligomers across different proteins. Immunostaining with the A11 antibody confirmed that the oligomeric amyloid beta quantum dots, like their native oligomeric counterparts, exhibited the correct oligomeric conformation. Additionally, positive staining with an antibody targeting amino acid residues verified the presence of the amyloid beta amino acid sequence at the N-terminal.
Assessing neuronal damage
Previous research has established that amyloid beta oligomers are capable of inducing neuronal and microglial damage through multiple mechanisms, including excitotoxicity, synaptic dysfunction, and structural changes. While examining immunoreactivity, investigators observed that exposure to oligomeric amyloid beta quantum dots and native spheroidal amyloid beta oligomers predominantly occurred alongside neuronal processes, with neuronal damage made evident through dendritic beading.
Dendritic beading is a morphological hallmark of dendritic injury. Under normal physiological conditions, dendrites—branch-like extensions of neurons—receive input from other neurons and transmit signals to the cell body. However, following injury, focal swelling appears along the dendritic shaft, causing the dendrites to resemble beads on a string, hence the term “dendritic beading.”
Furthermore, structural damage resulting from excitotoxicity and cellular stress responses has been documented in the presence of amyloid beta oligomers. The oligomeric amyloid beta quantum dots were found to trigger these neuroinflammatory hallmarks, including dendritic beading. Notably, this effect was specifically associated with the presence of the amyloid beta peptide, as control quantum dots lacking Aβ 1−16 functionalization exhibited little to no dendritic beading compared to vehicle-treated groups. Additionally, oligomeric amyloid beta quantum dots displayed other indicators of neuroinflammation, including excitotoxicity due to intracellular calcium dysregulation.
Effects on microglia
Amyloid beta can accumulate in both intracellular and extracellular spaces, where microglia play a crucial role in its phagocytic uptake and clearance. However, when the amyloid burden becomes excessive, microglial processing of amyloid beta oligomers can be disrupted, resulting in a pro-inflammatory response. To investigate whether amyloid beta quantum dots could trigger a similar neuroinflammatory reaction, researchers employed the mixed lineage kinase inhibitor URMC-099 to enhance amyloid uptake in microglia. Microglia exhibited an amyloid-specific phagocytic response to oligomeric amyloid beta quantum dots.
This study demonstrated the advantages of quantum dots in forming larger, spherical amyloid beta structures and provided the first visualization of cytotoxic amyloid oligomer phagocytosis involving larger, spheroidal oligomers. While glial cells have previously been shown to phagocytose monomeric and certain pre-fibrillar amyloid beta variants, evidence supporting the phagocytosis of oligomeric forms has been lacking.
Analyzing amyloid beta quantum dots with StressMarq products
Using quantum dots to construct larger, spherical amyloid beta structures offers several advantages over detecting native oligomers with antibodies. Quantum dots are exceptionally bright, making them ideal for imaging detection. Additionally, they can be modified to overcome specificity issues and size limitations often associated with the antibody conjugates traditionally used to detect dense oligomeric aggregates.
The amyloid beta quantum dots constructed by Chiang et al. closely resemble native spheroidal Aβ oligomers, as confirmed by direct comparison to StressMarq’s Amyloid Beta 1-42 Oligomers (catalog #SPR-488), and are capable of inducing key processes involved in neurodegeneration in both neuronal and glial cells. Notably, this study provides the first description of glial cell phagocytosis of larger, cytotoxic spheroidal amyloid oligomers.
The design and construction protocols established in this study lay a foundation for developing powerful tools to visualize molecular mechanisms underlying protein aggregation, ultimately advancing our understanding of interactions between different conformational forms of amyloid proteins.
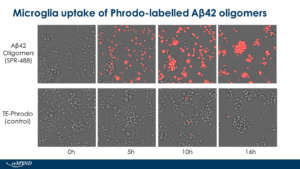
[Image from: StressMarq website] Live cell imaging of human iPSC-derived microglia uptake of StressMarq Amyloid Beta 1-42 Oligomers (catalog# SPR-488) conjugated to pHrodo dye.
Summary
Amyloid beta oligomers have been established as the most toxic and pathogenic form of amyloid beta, contributing to neuronal damage and the progression of Alzheimer’s disease. However, studying these oligomers has been challenging due to their structural variability and transient nature, leading to uncertainties regarding their causative effects.
The design and construction of quantum dot mimetics for larger, spherical oligomeric amyloid beta offer significant advancements in investigating their role in neuroinflammation. This innovative approach overcomes past technical challenges in isolating and studying amyloid beta oligomers, which have previously hindered the ability to generate direct evidence of their interactions with neuronal and glial cell types. Overall, these findings support the use of quantum dot mimetics for oligomeric amyloid beta, potentially providing critical insights into the dynamic interactions driving neurodegeneration.
Related StressMarq products
StressMarq is dedicated to providing high-quality reagents to support neurodegenerative research, offering an extensive range of oligomeric, fibrillar and monomeric protein preparations. Visit our website for more information, including the latest scientific publications using our specialized amyloid beta, tau, and alpha synuclein proteins for neurodegenerative disease research.
References
- Hybrid Amyloid Quantum Dot Nano-Bio Assemblies to Probe Neuroinflammatory Damage. Chiang, W., et al. ACS Chemical Neuroscience. 2024.
- Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Kayed, R., et al. Science. 2003.

Leave a Reply