A Novel Modifier of Alpha Synuclein-Induced Toxicity
Affecting approximately one million people in the United States alone, Parkinson’s disease (PD) is the most common movement disorder. Canadian actor Michael J. Fox has played a significant role in raising awareness of the disease, particularly the locomotor deficits experienced by patients which include stiffness, tremors, and coordination difficulties that worsen over time. In addition to motor symptoms, patients often experience sleep disturbances linked to circadian rhythm disruptions.
This progressive decline in motor function is driven by the loss of dopaminergic neurons, primarily due to the aggregation of alpha synuclein into insoluble protein inclusions known as Lewy bodies (LB). This aggregation induces oxidative stress, neuroinflammation, and neuronal cell death. Drosophila models of alpha synuclein aggregation serve as valuable tools for studying neurodegeneration, as they exhibit symptoms analogous to those seen in PD patients and share the same underlying disease mechanisms.
Recently, Lim et al. utilized the Drosophila alpha synuclein model to investigate the role of an enzyme involved in lipid metabolism in alpha synuclein cytotoxicity. The study found that silencing the mitochondrial enzyme glycerol 3-phosphate acyltransferase (GPAT) significantly suppressed Parkinson’s disease-related phenotypes in Drosophila, preventing dopaminergic neuronal loss, locomotion defects, and circadian rhythm disturbances. These findings highlight GPAT’s pivotal role in modulating alpha synuclein aggregation in Parkinson’s disease and introduce a promising new therapeutic target for mitigating disease progression.
Suppressing alpha synuclein neurotoxicity
Drosophila models have been instrumental in screening for genetic modifiers of neurodegeneration. Their ability to replicate complex neurophysiological traits seen in human patients—such as locomotor impairments caused by the progressive loss of dopaminergic neurons and disruptions in circadian rhythm—makes them highly valuable in neurodegenerative disease research. Additional advantages including their short life cycle, cost-effectiveness, and ease of handling, further establish Drosophila as an ideal model organism. These factors motivated researchers from Nanyang Technological University in Singapore (currently ranked 15th globally according to the QS World University Rankings 2025) to employ this model in their study.
Through a preliminary genetic screen, the mitochondrial isoform of GPAT, known as mino, was identified as a novel modifier of alpha synuclein neurotoxicity. This finding aligns with previous research showing that alpha synuclein interacts extensively with lipids. Overexpression of alpha synuclein in Drosophila led to significant declines in climbing ability, a common metric for assessing motor function. However, targeted depletion of mino/GPAT via RNA interference (RNAi) alleviated these deficits, demonstrating a protective effect against alpha synuclein-induced neurotoxicity. Additionally, reducing endogenous GPAT levels suppressed both dopaminergic neuronal loss and circadian rhythm disruptions. This effect was shown to be GPAT-specific, as GPAT overexpression reversed these protective benefits.
A key cellular mechanism in alpha synuclein pathology is neuron-glia crosstalk, which facilitates the spread of fibrillar aggregates and accelerates disease progression. Notably, GPAT silencing in both neurons and glial cells was necessary to mitigate the decline in daily locomotor activity caused by alpha synuclein overexpression. This highlights the crucial role of GPAT in modulating neurodegeneration and suggests its potential as a therapeutic target in Parkinson’s disease.
Downregulation of GPAT
Misfolding and aggregation of alpha synuclein into high molecular weight (MW) oligomers and fibrils are central to Parkinson’s disease pathology. Once aggregated, alpha synuclein accumulates into insoluble intraneuronal Lewy bodies, facilitating neurodegeneration by disrupting cellular functions, inducing mitochondrial damage, and impairing synaptic activity. Previous studies have emphasized the role of phospholipids in triggering early-stage alpha synuclein misfolding and aggregation. Given GPAT’s involvement in glycerolipid synthesis, it was crucial to determine whether GPAT influences the formation of pathogenic alpha synuclein fibrillar aggregates.
To investigate this, researchers employed Triton-free buffers along with the amine-reactive DTBP crosslinker to stabilize the native composition and organization of cellular protein complexes. Biochemical isolation and analysis revealed that GPAT downregulation specifically suppressed the formation of pathogenic high MW alpha synuclein oligomers. This effect was unique to GPAT, as RNA silencing of a related enzyme had no impact. Additionally, GPAT depletion did not affect lower MW alpha synuclein species such as dimers and monomers, further underscoring its selective role in promoting pathogenic aggregation.
Uncovering a novel modulator of alpha synuclein-induced cytotoxicity using StressMarq’s alpha synuclein constructs
In this study, researchers investigated the role of mitochondrial glycerol-3-phosphate acyltransferase (GPAT) in the formation of pathogenic alpha synuclein aggregates associated with PD. Utilizing primary mouse neurons treated with StressMarq’s Alpha Synuclein Pre-formed Fibrils (catalog #SPR-322 & catalog# SPR-324), it was observed that these fibrils induced the accumulation of Lewy body-like inclusions characterized by elevated levels of serine-129 phosphorylated alpha synuclein (pS129-αSyn), a common marker in Parkinson’s disease pathology. Notably, treatment with Alpha Synuclein Monomers (catalog# SPR-323) did not produce such inclusions, underscoring the pathogenicity of the fibrillar form.
StressMarq’s alpha synuclein fibrillar and monomeric alpha synuclein aggregates recapitulated the key molecular features of Parkinson’s disease, including the abnormal phosphorylation and aggregation of alpha synuclein into pathogenic LB inclusions. Importantly, pharmacological inhibition of GPAT significantly reduced the formation of these pathological pS129-αSyn inclusions, suggesting that therapeutic inhibition of GPAT activity may be a valuable tool in reducing alpha synuclein aggregation.
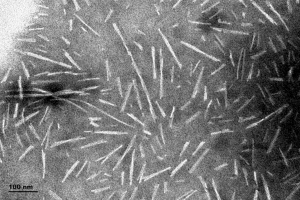
[Image from: StressMarq website] TEM imaging of Alpha Synuclein Pre-formed Fibrils (catalog# SPR-322).
Summary
Easily reproducible, cost-effective, and accurate models of PD are necessary for the continued investigation into the cellular and molecular factors that influence alpha synuclein neurotoxicity. In the study conducted by Lim et al., overexpression of alpha synuclein in Drosophila induced Parkinson’s disease pathology, including the loss of dopaminergic neurons.
Identification of the lipid enzyme GPAT as potent modifier of alpha synuclein cytotoxicity in Drosophila models enabled researchers to elucidate mechanims of GPAT inhibition in an in vitro mammalian model employing fibrillar and monomeric alpha synuclein constructs. As pharmacological inhibition of GPAT was demonstrated to ameliorate the pathogenic accumulation of alpha synuclein, the data presented in this study suggests that targeting disease-associated lipids may be a promising therapeutic intervention in Parkinson’s disease.
Related StressMarq products
StressMarq manufactures the most comprehensive and diverse range of alpha synuclein protein constructs for the neurodegenerative research field. Related tools for studying cell entry and localization include the Alpha Synuclein E114C Pre-formed Fibrils: ATTO 488 (catalog #SPR-518-A488) & Monomers (catalog #SPR-517-A488). Visit our website for more information, including the latest scientific publications using our specialized alpha synuclein, tau & amyloid beta protein constructs.
References
- Identification of glycerol 3-phosphate acyltransferase as a potent modifier of α-synuclein-induced toxicity. Lim, K.L. et al., Research Square [Preprint]. 2024.


Leave a Reply