Amyloid Beta Oligomers: Effects on Neurodegeneration
Neurodegenerative diseases are a group of disorders characterized by the progressive degeneration and loss of cells within the brain, resulting in cognitive decline, impairment of motor skills, and reduced functional capabilities. These diseases, which include Alzheimer’s, Parkinson’s, and Huntington’s disease, are among the most complex medical challenges of the 21st century. While the precise causes of many neurodegenerative diseases remain unclear, abnormal protein aggregation is a recurrent feature. In the case of Alzheimer’s disease (AD), amyloid beta oligomers have emerged as key contributors to neuronal dysfunction and disease progression.
Although amyloid plaques were historically recognized as an important facilitator of AD pathology, recent research suggests that amyloid beta oligomers—the small, soluble, and toxic aggregates of the amyloid beta protein—may be the driving force behind disease advancement. Enhanced understanding of these oligomeric proteins may facilitate the development of novel treatment strategies and early diagnostic tools.
Amyloid Beta Oligomers
Amyloid beta (Aβ) is a peptide derived from the cleavage of the amyloid precursor protein (APP), a membrane-bound protein involved in various cellular functions, including synapse formation, neural plasticity, antimicrobial activity, and mineral transport. Mutations in the APP gene have been linked not only to AD but also to metabolic disorders such as cardiovascular disease and type 2 diabetes. Although APP is found in many tissues and organs, it is predominantly located in the brain and spinal cord.
Under healthy physiological conditions, Aβ peptides are cleared from the brain by a diverse array of mechanisms. However, in Alzheimer’s disease, an imbalance between the rate of peptide production and clearance efficiency generates an excess accumulation of Aβ, which is able to aggregate into different forms.
Amyloid beta oligomers represent an intermediate state between monomeric (single peptide) Aβ and the large, insoluble plaques traditionally associated with Alzheimer’s disease pathophysiology. Unlike plaques, oligomers remain soluble and can readily spread throughout the brain, disrupting neuronal function. These small aggregates vary in size and structure, rendering them difficult to characterize, study, and target.

StressMarq offers Human Synthetic Amyloid Beta 1-42 Monomers (catalog# SPR-485), Oligomers (catalog# SPR-488) and Pre-formed Fibrils (PFFs) (catalog# SPR-487) as well as Human Amyloid Beta Pyroglutamate 3-42 PFFs (catalog# SPR-492) to support Alzheimer’s disease research.
Mechanisms of Toxicity
Synaptic Dysfunction: In its soluble oligomeric form, amyloid beta impairs neuronal communication by binding to synaptic receptors located at pre- and post-synaptic sites on neurons. Over time, their presence reduces long-term potentiation (LTP), a process essential for the maintenance of healthy learning and memory function. The resultant synaptic degradation severely impairs cognitive function and accelerates neurodegenerative processes.
Inhibition of Neurotransmission: Glutamate is a central excitatory neurotransmitter that supports cognitive function, learning, and memory. Research suggests that amyloid beta oligomers impair the function of key neurotransmitters, including glutamate and acetylcholine, worsening cognitive and functional decline. By disrupting glutamatergic signaling, Aβ oligomers induce excitotoxicity, where excessive glutamate levels trigger calcium overload and neuronal apoptosis. Similarly, reduced acetylcholine activity, a neurotransmitter vital for memory and cognition, further disrupts neural communication pathways.
Inflammatory Response: The accumulation of oligomeric Aβ aggregates may trigger inflammatory immune responses, leading to microglial and astrocyte activation. Initially, these glial cells attempt to clear toxic aggregates, but prolonged activation in response to persistent toxic amyloid beta exposure can result in the extended release of pro-inflammatory cytokines, driving chronic inflammation. This exacerbates pre-existing neuronal damage and enables neuronal degeneration. Furthermore, emerging research suggests that these oligomers may compromise the integrity of the blood-brain barrier (BBB), allowing harmful neurotoxic compounds to enter the brain and augment neuroinflammation.
Disruption of Cellular Homeostasis: Excess production of reactive oxygen species (ROS) due to interactions between amyloid beta oligomers and the neuronal cell membrane induces cellular oxidative stress and initiates harmful processes, including lipid peroxidation, DNA fragmentation, and protein degradation. These interactions also disrupt calcium homeostasis, an essential co-factor in cellular function. Additionally, these interactions are capable of impairing mitochondrial function, depleting neuronal energy supplies and amplifying synaptic loss.
StressMarq’s Amyloid Beta Oligomers
Cleavage of APP produces several forms of the Aβ peptide, with Aβ42 (a 42-amino acid proteolytic product) as the predominant form implicated in amyloid beta plaque formation in various neurodegenerative diseases. StressMarq’s Amyloid Beta 1-42 Oligomers (catalog# SPR-488) enable researchers to generate cutting-edge, reproducible models of Aβ aggregation in order to study its toxic effects and replicate disease pathology with precision.

Survival of rat primary cortical neurons 14 days after treatment with different concentrations of Human Synthetic Amyloid Beta 1-42 (A) Monomers (catalog# SPR-485), (B) Oligomers (catalog# SPR-488) or (C) Pre-formed Fibrils (catalog# SPR-487), quantified by MAP2 positive neurons and expressed as a percentage of control.
Human Synthetic Amyloid Beta 1-42 Oligomers: StressMarq’s Aβ42 Oligomers are generated from amyloid beta peptide 1-42. These synthetic proteins present as globular oligomers when observed under TEM and AFM, and produce a distinct dimer/trimer and oligomer signal on a Western Blot when visualized with an anti-amyloid beta oligomer antibody. In toxicity experiments conducted on rat primary cortical neurons, these oligomers exhibit dose-dependent toxicity.
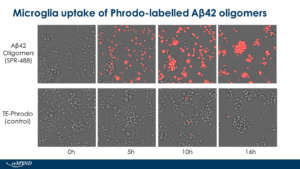
Human iPSC-derived microglia uptake of StressMarq Amyloid Beta 1-42 Oligomers (catalog# SPR-488) conjugated to pHrodo dye. Live cell imaging performed by reMYND (Leuven, Belgium) using Ex560 Em585.
Targeting Oligomeric Amyloid Beta
The study of neurodegenerative diseases continues to evolve, with current efforts focused on developing improved diagnostic tools and therapeutic interventions. Biomarkers that detect toxic amyloid beta oligomers in patient cerebrospinal fluid (CSF) or blood samples could enable earlier diagnosis, allowing earlier intervention to slow cognitive and motor decline. Additionally, advanced imaging techniques may allow researchers to observe oligomer formation in living patients, providing crucial insights into disease progression.
Several experimental approaches are in development to address these challenges:
- Antibody-based therapies, such as monoclonal antibodies that preferentially recognize and clear toxic oligomers without disrupting normal physiological amyloid beta.
- Small-molecule inhibitors that prevent the aggregation of monomeric Aβ into oligomers.
- Enhancing clearance mechanisms through immunotherapy or by upregulating the brain’s natural degradation pathways.
Despite the growing recognition of amyloid beta oligomers as key players in Alzheimer’s disease, their transient and dynamic nature makes them difficult to isolate and characterize. As oligomers inherently vary in structure, developing drugs to neutralize or prevent their formation without affecting normal brain function is a complex process that requires the use of high-quality research tools.
Specialized Protein Constructs for Neurodegenerative Disease Research
By utilizing a well-characterized oligomeric amyloid beta preparation such as StressMarq’s Amyloid Beta 1-42 Oligomers (catalog# SPR-488) in neurodegenerative disease models, scientists can gain deeper insights into the molecular mechanisms of Alzheimer’s disease and advance precise therapy development.
As an experienced manufacturer of fibrillar and oligomeric preparations for in vitro and in vivo research models, StressMarq is proud to offer a diverse range of innovative amyloid beta, tau, and alpha synuclein protein constructs for neurodegenerative disease research. Researchers worldwide can access these reagents through our international distributor network.
Rigorous Quality Control
StressMarq’s quality control testing for our amyloid beta proteins includes:
- TEM/AFM imaging to verify fibril formation
- SDS-PAGE to ensure protein purity
- Sterility check
- Endotoxin testing
Product Citations
StressMarq’s monomeric, fibrillar and oligomeric amyloid beta preparations have been cited in recent scientific research publications. Certain products have also been validated in various applications by research collaborators.
References
- Neurotoxic soluble amyloid oligomers drive Alzheimer’s pathogenesis and represent a clinically validated target for slowing disease progression. Tolar, M. et al. Int J Mol Sci. 2021.
- The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Karran, E. & De Strooper, B. Nature Reviews Drug Discovery. 2022.
- Aβ oligomer toxicity-reducing therapy for the prevention of Alzheimer’s disease: Importance of the Nrf2 and PPARγ pathways. Araki, W. Cells. 2023.

Leave a Reply