Biomarker Analysis Via Infrared Detection of Alpha Synuclein
Synucleinopathies are a group of neurodegenerative diseases marked by the abnormal accumulation of alpha synuclein in the brain. Prominent examples include Parkinson’s disease (PD), a neurodegenerative movement disorder that affects approximately 8.5 million people worldwide according to recent estimates from the World Health Organization (WHO), and Multiple System Atrophy (MSA).
Under normal physiological conditions, alpha synuclein exists in a monomeric form. In PD pathology, it progressively misfolds into neurotoxic oligomeric and fibrillar aggregates that accumulate into structures known as Lewy bodies (LBs). This irregular assembly process disrupts neuronal function and leads to the degeneration of dopaminergic neurons across the brain. As dopamine is a critical component in the regulation of movement, changes to dopamine levels disrupt motor function, resulting in bradykinesisa, muscular rigidity, and resting tremors. Disease progression corresponds with increasingly pronounced cognitive impairment, and is driven by the intracellular spread of alpha synuclein aggregates between neurons and astrocytes throughout different regions of the brain.
Similarly, MSA is a rare, rapidly progressive and fatal neurodegenerative disorder that shares several clinical features with Parkinson’s disease. Both diseases exhibit overlapping neuropathological traits, most notably the accumulation of aggregated alpha synuclein. In MSA, these abnormal aggregates form within glial cytoplasmic inclusions (GCIs), which are located in oligodendrocytes of the central nervous system (CNS).
Alternative detection methods
The defining neuropathological feature of synucleinopathies is the abnormal accumulation of alpha synuclein. In recent years, seed amplification assays (SAAs) have emerged as valuable tools for detecting aggregated and misfolded alpha synuclein in bodily fluids, particularly cerebrospinal fluid (CSF) from the CNS.
Real-Time Quaking-Induced Conversion (RT-QuIC) assays are a category of SAAs that exploit the ability of misfolded, aggregated alpha synuclein to act as a “seed” that drives the aggregation of monomeric alpha synuclein into neurotoxic aggregates. These diagnostic methods have been validated to detect fibrillar and oligomeric alpha synuclein in CSF across various stages of disease progression, achieving an accuracy exceeding 90%.
However, these assays are not without limitations. Variability in experimental parameters and differences in the quality of monomeric alpha synuclein used can introduce inconsistencies, making reproducibility across laboratories challenging. Other detection techniques, such as Positron Emission Tomography (PET) imaging, are viable alternatives but remain in the early stages of development and are prohibitively expensive. As such, additional methods for detecting alpha synuclein aggregation are earnestly being investigated.
Recent research by Schuler et al. highlights the development of a diagnostic test using infrared (IR) spectroscopy. IR spectroscopy analyzes the protein’s secondary structure by examining the vibrational states of amide bonds. Within the context of alpha synuclein, this technique can distinguish between protein conformations. Monomeric alpha synuclein exhibits alpha-helical structures, while neurotoxic aggregated forms demonstrate a β-sheet-enriched structure. The recent findings suggest that IR spectroscopy can effectively detect neurotoxic forms of alpha synuclein in CSF samples from patients with PD and other synucleinopathies, offering a promising new diagnostic approach.
Probing alpha synuclein structures
Infrared (IR) spectroscopy is a label-free technique that examines the secondary structure of proteins by analyzing the vibrational changes of amide bonds. When a protein is exposed to IR light, these bonds, which link amino acids, undergo stretching and vibrations. The amide I bond, representing the C=O stretching of the peptide backbone, produces peaks at specific wavelengths depending on the protein’s secondary structure. This capability makes IR spectroscopy particularly advantageous for analyzing protein conformations and their implications in disease research.
IR sensor detection of biomarkers
Next, the feasibility of using the immuno-infrared sensor to measure alpha synuclein aggregates in CSF as biomarkers for PD was assessed. Cerebrospinal fluid is a well-established medium for detecting aggregated proteins associated with neurodegenerative disorders, making it a valuable resource for diagnosis and monitoring disease progression. However, albumin—the most abundant protein in CSF—can interfere with experimental setups due to its tendency to bind to other proteins non-specifically. Thus, it was essential to validate the performance of the immuno-infrared sensor in the context of analyzing CSF samples.
Experiments were subsequently conducted using the immuno-infrared sensor, both with and without the capture antibody. The experimental results established that the alpha synuclein capture antibody was highly effective in specifically binding to all conformations of alpha synuclein, with the majority of the protein present in the CSF being successfully captured and analyzed. Additionally, the capture antibody and alpha synuclein demonstrated robust binding interactions, with EC50 values in the sub-nanomolar range, further confirming the sensor’s efficiency and specificity.
Alpha synuclein biomarker detection
In the final phase of the study, researchers tested samples from patients diagnosed with synucleinopathies. CSF was collected from independent patient populations with various neurodegenerative diseases. Patients diagnosed with Parkinson’s disease and MSA showed elevated levels of aggregated alpha synuclein. In contrast, samples from patients with tauopathies exhibited minimal levels of aggregated alpha synuclein. The group of patients with Parkinson’s disease and MSA, categorized as high alpha synuclein aggregate expressers, was clearly distinguishable from those with diseases associated with low alpha synuclein aggregation. The strong predictive performance of the immuno-infrared sensor was reflected in a high Area Under the Curve (AUC) value.
To further refine the analysis, the researchers employed a two-threshold system instead of a single cutoff. This allowed patients to be categorized into three groups based on alpha synuclein aggregation levels: low, intermediate, and high. This approach better represented the spectrum of healthy controls and individuals with confirmed synucleinopathies. When comparing patients with Parkinson’s disease and MSA to healthy controls, the immuno-infrared sensor demonstrated exceptional diagnostic accuracy, achieving a sensitivity of 97% and a specificity of 92%.
Developing biomarker detection methods using StressMarq products
The research findings by Schuler et al. mark a significant milestone as the first successful detection of alpha synuclein aggregates in CSF from patients with Parkinson’s disease. The immuno-infrared sensor developed in this study offers distinct advantages over other assays, as it can detect all conformations of alpha synuclein—ranging from non-aggregated, physiological monomers to neurotoxic aggregates, including oligomers and fibrils. StressMarq’s Alpha Synuclein Monomers (catalog# SPR-321), Alpha Synuclein Oligomers (catalog# SPR-466), and Alpha Synuclein Pre-formed Fibrils (catalog# SPR-322) were instrumental in creating a diagnostic tool capable of classifying alpha synuclein conformations for biomarker detection.
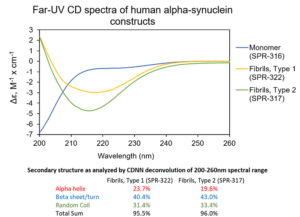
[Image from: StressMarq website] Visualizing fibrillar structures through UV-circular dichroism of StressMarq’s Alpha Synuclein Pre-formed Fibrils (catalog# SPR-322).
Summary
Seed amplification assays are highly useful for detecting the presence of fibrillar aggregates. Scientists set out to develop the immuno-infrared sensor described in this study in order to expand the detection range of the conventional SAA to include oligomeric and monomeric alpha synuclein in addition to its fibrillar conformation. This advancement reveals a diagnostic assay able to detect all conformations of alpha synuclein, with the capability to detect protein misfolding in an unmodified CSF matrix.
Overall, the findings provide a valuable diagnostic tool for Parkinson’s disease biomarkers. Further advancements could enable the integration of multiplexing features into a single platform. For example, the detection of numerous neurodegenerative biomarkers simultaneously would be immensely useful when different pathologies co-exist or clinical symptoms overlap, such as in PD and Multiple System Atrophy.
Related StressMarq products
StressMarq is dedicated to delivering high-quality reagents to advance neurodegenerative disease research, offering a range of oligomeric, fibrillar and monomeric protein preparations of neurodegenerative proteins, including alpha synuclein. The Alpha Synuclein A53T Mutant Pre-formed Fibrils (catalog# SPR-326) harbour a point mutation linked to a familial early-onset form of Parkinson’s disease. The study of different aggregation states in disease progression is facilitated by the Kinetically-Stable Alpha Synuclein Oligomers (catalog# SPR-484). Visit our website for more information, including the latest scientific publications using our specialized alpha synuclein, tau and amyloid beta protein constructs.
References
- Alpha-synuclein misfolding as a fluid biomarker for Parkinson’s disease and synucleinopathies measured with the iRS platform. Schuler, M. et al. medRxiv [Preprint]. 2024.

Leave a Reply