CDK14 Inhibition Decreases Alpha Synuclein Neurotoxicity
Parkinson’s disease is a progressively debilitating disease estimated to affect over 10 million individuals worldwide. Patients with Parkinson’s disease (PD) often present with motor symptoms such as bradykinesia (slowness of movement) and rigidity, and lesser-known non-motor symptoms such as constipation and sleep disturbances. An additional motor deficit commonly associated with increasing severity of the disease is a decline in handgrip strength. Alpha synuclein is a key protein associated with Parkinson’s disease pathology. Highly phosphorylated and abnormally aggregated forms of alpha synuclein accumulate into intraneuronal inclusions called Lewy bodies (LB), that contribute to neurotoxicity and neurodegeneration.
As increased abnormal alpha synuclein levels are linked to Parkinson’s disease pathogenesis, reducing overall levels of aberrantly phosphorylated forms of this protein is a therapeutic approach quickly gaining traction. By identifying a kinase able to modify alpha synuclein levels, a collaborative study between the University of Ottawa Brain and Mind Research Institute and several research institutions, led by Parmasad et al., describes how genetic and pharmacological reduction of cyclin-dependent kinase 14 (CDK14) can mitigate alpha synuclein pathology. The study results provide the impetus for investigating CDK14 inhibitors as potential therapeutics for Parkinson’s disease.
Alpha synuclein fibril-induced Parkinson’s disease mouse model
Previous research using a pooled RNA interference (RNAi) screen to elucidate “druggable” modifiers of alpha synuclein levels in Drosophila identified CDK14 as a factor of interest. Subsequently, a multi-institutional research collaboration began in order to elucidate whether genetic and pharmacological inhibition of CDK14 could reduce alpha synuclein pathology and Parkinson’s disease phenotype in mice and human models.
It has been observed that CDK14 and the alpha synuclein gene (SNCA) share similar gene expression patterns in the murine brain, specifically, the hippocampus and substantia nigra (SN). To study the effect of CDK14 silencing in the context of alpha synuclein pathology, CDK14 was absent in the alpha synuclein pre-formed fibril-induced Parkinson’s disease mouse model. Here, injection of StressMarq’s Mouse Alpha Synuclein Pre-formed Fibrils (catalog# SPR-324) or Human Alpha Synuclein Pre-formed Fibrils (catalog# SPR-322) resulted in the loss of dopaminergic neurons in the SN — the defining pathological hallmark of Parkinson’s disease — alongside mild motor impairments.
Moreover, wild-type alpha synuclein fibril-treated mice also suffered from weakness in their forelimbs, as measured by the grip strength test. In contrast, CDK14 silencing alleviated the loss of dopaminergic neurons in the SN, and prevented mice from developing grip strength deficits.
Reduction in the pathological buildup of phosphorylated forms of alpha synuclein
Phosphorylated forms of alpha synuclein, specifically pS129, have been observed to promote aggregation and LB formation. Moreover, elevated levels of this pathological marker have been identified in patients with PD. This was determined through comparison with a control of healthy patient population. Examining pS129 alpha synuclein-positive cells from all murine brain regions showed that CDK14 ablation diminished pS129 alpha synuclein-positive pathology. The protective effect was replicated with human dopaminergic neurons taken from a patient harbouring a Parkinson’s disease-associated mutation (A53T).
In addition, a closer inspection of regions of the brain in the alpha synuclein pre-formed fibril-induced PD mouse model revealed surprising results. Regions distal from the injection site of alpha synuclein demonstrated more pronounced alpha synuclein pathology in comparison with anatomical areas in closer proximity to the injection site. One explanation behind this distal pathology was that loss of CDK14 controlled the pathological spread by reducing the cell-to-cell propagation of alpha synuclein.
CDK14 pharmacological inhibition in alpha synuclein transgenic mouse model
To conclude, the researchers assessed whether pharmacological inhibitors of CDK14 had the same effect as the genetic reduction of CDK14. The researchers employed the covalent inhibitor (FMF-04-159-2), which is often used to target kinases by binding and forming covalent bonds with the target. Human neurons treated with the CDK14 inhibitor revealed a dose-dependent reduction in total alpha synuclein levels.
For in vivo experiments, a transgenic mouse model that overexpressed human alpha synuclein with a PD-associated mutation (A53T) was employed. As the background of the mice carried a partial allele of mouse SNCA, only human mutant alpha-synuclein was expressed, and all other alpha synuclein expression was eliminated. The CDK14 inhibitor was able to reduce human alpha synuclein (mutated A53T) protein levels without inducing any distress or pain in the mice.
StressMarq products employed in Parkinson’s disease mouse model
Gene multiplications and point mutations (A53T) of the alpha synuclein gene (SNCA) can cause overexpression and phosphorylation of alpha synuclein, which contribute to neurotoxicity. Therefore, decreasing the total level and phosphorylation of alpha synuclein is an attractive therapeutic strategy to treat Parkinson’s disease. Inhibition of CDK14 kinase appeared to have a protective role and prevented the buildup of pathological forms of phosphorylated alpha synuclein. Silencing CDK14 allowed the Parkinson’s disease mouse model to avoid developing motor deficits.
Futhermore, pharmacological CDK14 inhibition decreased total and pathologically aggregated alpha-synuclein in the a-Syn humanized mice and human neurons. The evidence from this study suggests that CDK14 is a novel therapeutic target for Parkinson’s disease. The pathological features of Parkinson’s disease can be recapitulated by injecting StressMarq’s Mouse Alpha Synuclein Pre-formed Fibrils (catalog# SPR-324) or Human Alpha Synuclein Pre-formed Fibrils (catalog# SPR-322) in a mouse model.
Fibril-treated mice exhibit motor symptoms such as limb weaknesses and accumulation of phosphorylated alpha synuclein in neurons. Parkinson’s disease mouse models are indispensable in the search for modulators of the disease. In conclusion, the data presented in the study suggest that the inhibitory effects of CDK14 may be a viable pre-clinical strategy for treating Parkinson’s disease.
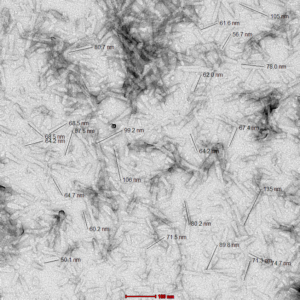
Figure 1. TEM of Mouse Recombinant Pre-formed Fibrils (Type 1) (catalog# SPR-324). Image: 100kx magnification.
Related StressMarq products
StressMarq manufactures a range of monomeric, oligomeric and fibrillar mouse and human alpha synuclein protein constructs for neurodegenerative disease research. The A53T missense point mutation, where alanine is replaced by threonine at the 53rd amino acid, is linked with early-onset Parkinson’s disease. StressMarq’s Human A53T Mutant Alpha Synuclein Pre-formed Fibrils (catalog# SPR-326) and Monomers (catalog# SPR-325) carry this clinically-important A53T mutation. Please visit our website for more information on our alpha synuclein products.
References
- Genetic and pharmacological reduction of CDK14 mitigates synucleinopathy. Parmasad, L.A. et al. Cell Death & Disease. 2024.
- A druggable genome screen identifies modifiers of α-synuclein levels via a tiered cross-species validation approach. Rousseaux M.W.C., et al. J Neurosci. 2018.

Your blog is a constant source of inspiration for me. Your passion for your subject matter shines through in every post, and it’s clear that you genuinely care about making a positive impact on your readers.