Chimeric Antigen Receptors in Alzheimer’s Disease Therapy
Chimeric antigen receptor (CAR) T-cell therapy, a form of immunotherapy, has demonstrated remarkable effectiveness in treating certain types of cancer. By re-engineering surface proteins found on white blood cells known as T-cells, CAR-T therapy enables these immune cells to direct their phagocytic activity against tumours. This innovative therapy has proven more effective, in some instances, than standard treatments for advanced cancers, and is approved by the Food and Drug Administration (FDA) for leukaemia, lymphoma, and myeloma. More recently, the CAR gene targeting proteins on cancer cells have been expressed in macrophages (CAR-M) to increase their phagocytotic and tumour-killing capacity. Engineered CAR-Ms have demonstrated advantages over CAR-Ts in the homing and infiltration of solid tumours. A new study published in JCI Insights explores the potential application of CAR-M therapy in diseases that are growing in prevalence and remain difficult to treat, such as Alzheimer’s disease.
The accumulation of amyloid beta is a crucial catalyst for the progression of Alzheimer’s disease. Insoluble beta sheets initially form, before further aggregating into amyloid fibrils and plaques. These plaques activate neurotoxic cascades, resulting in tau hyperphosphorylation and aggregation into neurofibrillary tangles (NFTs) that contribute to neurotoxicity and neurodegeneration. Researchers from Washington University in St. Louis have shown that CAR-M technology could potentially be harnessed to degrade pathogenic amyloid beta plaques.
CAR-Ms bind and resorb amyloid beta in culture
In order to target pathogenic amyloid beta fibrils and plaques, researchers first designed unique CAR-M constructs — known as amyloid beta CAR-Ms — using an FDA-approved drug indicated for the treatment of Alzheimer’s disease, Aducanumab, in a scFv (single-chain fragment variable) format. Following the retroviral expression of the amyloid beta CAR construct in macrophages, CAR function was assessed by analysing the uptake of StressMarq’s Human Synthetic Amyloid Beta 1-42 Peptide (catalog# SPR-485) by flow cytometry after labelling with Alexa Fluor 488. The observations at this stage were encouraging: the amyloid beta CAR-Ms displayed the ability to bind and resorb the monomeric amyloid beta peptide in culture, unlike their corresponding CAR-M controls, which targeted the EphA2 receptor.
The uptake of amyloid beta was further confirmed by monitoring phagocytic engulfment of the neurotoxic protein by the amyloid beta CAR-Ms, using confocal microscopy. LAMP-1 colocalization further indicated that amyloid beta was degraded and trafficked to lysosomal compartments in the amyloid beta CAR-Ms.
Furthermore, the amyloid beta CAR-Ms were able to take up monomeric, oligomeric, and fibrillar amyloid beta proteins. This was concluded by measuring the uptake of StressMarq’s monomeric Amyloid Beta 1-42 Peptide (catalog# SPR-485), Amyloid Beta 1-42 Oligomers (catalog# SPR-488) and Amyloid Beta Pyroglutamate 3-42 Pre-formed Fibrils (catalog# SPR-492).
In addition to recognizing amyloid beta-associated antigen presentation, the ideal CAR structure should also contain an intracellular cytoplasmic domain, which is indispensable for cellular signalling functions. The amyloid beta CAR-Ms contained a phagocytic common γ chain of the Fc receptor (FcRγ). Examination of downstream molecules modulated by these amyloid beta CAR-Ms confirmed that the CAR structure was functional, through the upregulation of co-stimulatory molecules such as CD40 and CD86.
Ex vivo reduction of amyloid beta plaque load by CAR-Ms
Next, the ex vivo effects of CAR-T therapy were assessed in the APP/PS1 mouse model of Alzheimer’s disease. Commonly utilized for studying Alzheimer’s disease pathology, this model exhibits robust amyloid beta production that is easily identifiable in brain slices. Incubation of the amyloid beta CAR-Ms on brain slices from the APP/PS1 mouse model were able to significantly reduce plaque burden.
One limitation of the first generation of CAR-Ms was the limited persistence of cells when injected intrahippocampally into the APP/PS1 mouse model. Indeed, the limited persistence of CAR immunotherapy poses a challenge for patient treatment and is the leading cause of T-cell exhaustion.
Amyloid beta CAR-Ms expressing M-CSF reduce hippocampal plaque load
Researchers subsequently aimed to improve upon the first generation of amyloid beta CAR-Ms, and provide a more efficacious alternative with enhanced in vivo stability. As the amyloid beta CAR-Ms required exogenous cytokines for survival, a second generation of cells was developed that secreted macrophage colony-stimulating factor (M-CSF) — a cytokine that promotes macrophage maturation. Additionally, prior to introducing the second-generation amyloid beta CAR-Ms, endogenous microglia were reduced in the hippocampi of aged APP/PS1 mice through the use of pre-conditioning treatments.
Despite the neuroprotective role of microglia in Alzheimer’s disease, they are also capable of inducing a chronic pro-inflammatory response that can lead to a detrimental cycle of neurotoxicity and neuronal damage. In order to reduce endogenous microglial levels, a low-dose of the chemotherapy drug Busulfan was introduced in tandem with the CSF-1 inhibitor PLX5622. This pre-conditioning treatment, in combination with the second-generation amyloid beta CAR-Ms, proved successful. Neuronal cells demonstrated improved survival outcomes alongside a significantly reduced amyloid beta plaque load in vivo.
Utilizing StressMarq products in the development of CAR-Ms
CAR T-cell immunotherapy has proven to be highly successful in cases of leukaemia and lymphoma, with observed remission rates of as high as 93%2. The study by Kim et al. successfully evaluated whether CAR-M therapy could prove beneficial within the context of Alzheimer’s disease. As this neurodegenerative disease is increasingly prevalent in the ageing population, scientists are continuously seeking novel and promising therapeutic avenues. StressMarq’s monomeric Amyloid Beta 1-42 Peptide (catalog# SPR-485), Amyloid Beta 1-42 Oligomers (catalog# SPR-488) and Amyloid Beta Pyroglutamate 3-42 Pre-formed Fibrils (catalog# SPR-492) enabled researchers to examine phagocytic uptake of amyloid beta by genetically-engineered amyloid beta CAR-Ms, that were then refined into more efficient second-generation iterations.
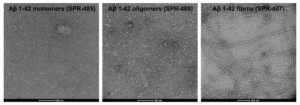
TEM of Human Synthetic Amyloid Beta 1-42 monomers (catalog# SPR-485, left), Oligomers (catalog# SPR-488, middle) and Pre-formed Fibrils (catalog#SPR-487, right). Images acquired at 80 Kv on carbon coated 400 mesh copper grids using phosphotungstic acid and uranyl acetate stain. Scale bar = 100 nm.
Summary
One of the earliest toxic events in the pathogenesis of Alzheimer’s disease is the aggregation of amyloid beta to form fibrils that deposit into amyloid plaques. Kim et al. discovered that CAR macrophage immunotherapy exhibits the ability to reduce amyloid beta accumulation, which could be crucial in preventing disease progression past the early stages of Alzheimer’s disease. The data presented in this study supports the adaptation of CAR T-cell immunotherapy beyond cancer treatment. CAR-M therapy possesses the ability to reduce the pathogenic accumulation of neurotoxic amyloid beta in Alzheimer’s disease, opening the door to new methods of combatting neurodegenerative diseases.
Related StressMarq products
StressMarq is the market leader of unique fibrillar, oligomeric, and monomeric protein preparations for neurodegenerative disease research. Visit our website for details on our diverse range of tau, amyloid beta, and alpha synuclein protein constructs, and a list of other scientific publications where our products have been used.
References
- Chimeric antigen receptor macrophages target and resorb amyloid plaques. Kim, A.B. et al. JCI Insight. 2024.
- Chimeric antigen receptor T-cell therapy clinical results in pediatric and young adult B-ALL. DiNofia, A.M. & Maude, S.L. Hemasphere. 2019.


Leave a Reply