Detecting Oligomeric Tau using a Novel ELISA
Approximately 47 million people worldwide are living with Alzheimer’s disease (AD), a progressive neurodegenerative disorder characterized by declining memory and cognitive function. While the accumulation of amyloid beta plaques is considered to be a central event in disease progression, tau pathology and neuroinflammation also play critical roles in exacerbating neurodegeneration. Over time, these pathological processes contribute to the cognitive and behavioral impairments associated with Alzheimer’s disease.
Under normal physiological conditions, tau is a monomeric, intrinsically disordered protein that stabilizes microtubules. In AD, tau undergoes post-translational modifications, particularly hyperphosphorylation, leading to structural changes that result in oligomeric and fibrillar tau species. These aggregates are able to form intracellular neurofibrillary tangles (NFTs), a key hallmark of tau pathology. Among these, soluble tau oligomers are considered the most neurotoxic, as they facilitate disease progression by promoting further aggregation of physiological monomeric tau.
The progression of tau pathology follows distinct patterns in the brain, classified by the Braak staging system, which consists of six stages (I–VI). These stages correspond to the spread of NFTs through neuronal networks and strongly correlate with neuronal loss and cognitive decline. Since neurotoxic oligomeric tau plays a crucial role in initiating and propagating tau aggregation, analyzing these oligomers across different Braak stages is essential. Utilizing an enzyme-linked immunosorbent assay (ELISA) to detect oligomeric tau could provide valuable insights into disease progression, improve early diagnosis, and pave the way for more effective treatment strategies for Alzheimer’s disease.
Challenges in detecting oligomeric tau
The isolation and analysis of oligomeric and higher molecular weight (MW) forms of tau have been challenging due to their inherent instability. Traditionally, experimental techniques such as Western blot analysis have been used to separate and distinguish protein conformations based on their MW. However, these methods often involve the use of sodium dodecyl sulfate (SDS) and reducing agents like dithiothreitol (DTT) or 2-mercaptoethanol, which can disrupt oligomeric and fibrillar tau structures. As a result, accurately detecting and assessing the contributions of these pathological tau species has been difficult.
To overcome this limitation, alternative techniques such as size-exclusion chromatography, density gradient centrifugation, and ELISA employ physiological buffers and avoid harsh reducing agents. These approaches enable a more accurate examination of the pathological significance of oligomeric and fibrillar tau species in samples from Alzheimer’s disease patients.
In a study by Fukumoto et al. published in The FASEB Journal, researchers from APRINOIA Therapeutics Inc. developed an ELISA using a novel mouse monoclonal antibody, APNmAb005. This assay successfully detected significant increases in oligomeric tau in frontal lobe samples from individuals with advanced Alzheimer’s disease (Braak stages V and VI), which are associated with severe cognitive decline. Moreover, the assay effectively distinguished tau oligomers from other neurodegenerative proteins, including alpha synuclein and amyloid beta. By specifically targeting tau oligomers, this ELISA holds promise as a diagnostic tool for Alzheimer’s disease and other neurodegenerative conditions linked to tau pathology.
Development of a novel ELISA
To initiate the study, researchers designed an ELISA protocol for the specific detection of neurotoxic tau oligomers. A key requirement for this assay was the use of a highly specific antibody. For this purpose, the team employed their previously generated monoclonal antibody APNmAb005, whose specificity is detailed in an initial study currently under peer review. Its ability to preferentially bind high MW oligomeric tau was previously validated through dot blot analysis. Additionally, recognized tau oligomers in samples from patients with neurodegenerative diseases, including Alzheimer’s disease.
Next, it was essential to optimize both the sample and assay conditions to preserve the disease-relevant conformations of tau. Samples were prepared in PBS buffer containing heparin, a structural analogue of heparan sulfate, which is naturally found in the extracellular matrix. As a highly negatively charged polysaccharide, heparin helped maintain the β-sheet-rich structures of the tau oligomers.
Sample preparation involved size-exclusion chromatography and sucrose density gradient centrifugation to separate different tau conformations by size. Oligomeric tau species were found in fractions corresponding to a MW of 2000 kDa and higher, with more extensively oligomerized tau species present. Morphological analysis confirmed the presence of oligomeric tau without contamination from larger fibrillar aggregates. The APNmAb005-ELISA specifically detected these oligomeric tau forms while excluding monomeric tau. Standard curve analysis using recombinant high-MW tau demonstrated the assay’s high precision, with a dose-response curve showing strong linearity (R² > 0.99) and a low detection limit (0.3 pg/well).
Validation of ELISA specificity using StressMarq’s neurodegenerative proteins
Summary
Neurodegenerative disease research is challenging due to the diverse conformational states that neurodegenerative proteins can adopt. Analysis of neurotoxic tau oligomers, which accumulate to form neurofibrillary tangles (NFTs), is further complicated by the susceptibility of these structures to alteration under harsh chemical conditions.
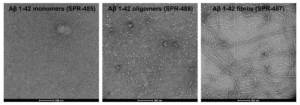
This research presents the development of a highly specific and sensitive ELISA capable of identifying high MW oligomeric tau. In cross-reactivity studies, the assay demonstrated exceptional specificity, showing no detection of other β-sheet-rich proteins in either oligomeric or fibrillar conformations. Notably, the ELISA did not recognize StressMarq’s Amyloid Beta 1-42 Oligomers (catalog #SPR-488), Amyloid Beta 1-42 Pre-formed Fibrils (catalog #SPR-487), Alpha Synuclein Kinetically Stable Oligomers (catalog #SPR-484), or Alpha Synuclein Pre-formed Fibrils (catalog #SPR-322). The study by Fukumoto et al. has introduced a novel research tool capable of distinguishing patient samples based on the progression of Alzheimer’s disease. Its potential to enhance diagnostic accuracy could lead to earlier detection of disease pathology and improved treatment strategies.
Related StressMarq Products
StressMarq manufactures a wide range of neurodegenerative proteins in different conformations, providing researchers with the high-quality tools needed to develop robust assays for neurodegenerative disease studies. Visit our website for more information, including the latest scientific publications using our specialized tau, amyloid beta and alpha synuclein proteins for neurodegenerative disease research.
References
- High-molecular-weight oligomer tau (HMWoTau) species are dramatically increased in Braak-stage dependent manner in the frontal lobe of human brains, demonstrated by a novel oligomer Tau ELISA with a mouse monoclonal antibody (APNmAb005). Fukumoto, H. et al., FASEB J. 2024.


Leave a Reply