Dynamic Mechanisms of Microglial Tau Endocytosis
Tauopathies are a diverse group of neurodegenerative diseases characterized by the abnormal aggregation and spread of the tau protein in the brain. At present, there are at least 27 known tauopathies, with Alzheimer’s disease and frontotemporal dementia being the most prominent. In Alzheimer’s disease, tau undergoes a sequence of misfolding, aggregation, and hyperphosphorylation, leading to the accumulation of toxic tau aggregates. This process ultimately results in neuronal damage and death and is further exacerbated by the propagation of tau to different brain regions.
The extent of tau aggregation is strongly correlated with symptom severity, as patients experience progressive memory loss and cognitive decline as tau aggregates form and spread throughout the brain. This propagation occurs in a prion-like manner, where neurotoxic tau seeds—composed of oligomeric and fibrillar tau—induce the misfolding of physiologically normal tau upon entering recipient cells. Additionally, tau can be secreted extracellularly, either as a free protein or enclosed within vesicles, further facilitating its spread throughout the brain.
While microglia, the brain’s resident immune cells, can release tau seeds that exacerbate misfolding in recipient cells, they also have the ability to internalize and degrade tau, potentially mitigating its toxic propagation. Microglial endocytosis is believed to be the primary mechanism for tau seed uptake and clearance, making it a key focus of neurodegenerative disease research. Scientists worldwide are now investigating how endocytic pathways may contribute to disease progression and whether targeting these mechanisms could offer therapeutic potential.
Endocytic regulation
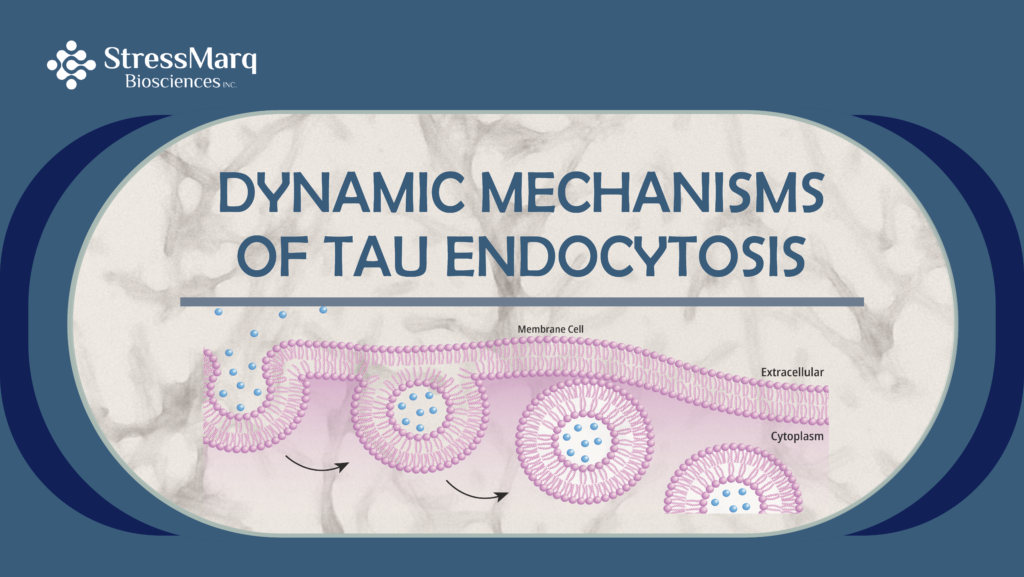
Previous research has identified endocytosis as the primary mechanism by which tau seeds are taken up and displaced throughout the brain. The endocytic process can be classified into four distinct pathways, with most studies focing on tau uptake and spread within neurons. Two main tau endocytosis pathways have been identified under these conditions: clathrin-mediated endocytosis and macropinocytosis.
Macropinocytosis is a regulated process that enables the non-specific fluid uptake of solutes, nutrients, and antigens. These droplets of medium are then trafficked throughout the endocytic system. In contrast, clathrin-mediated endocytosis is a more selective mechanism that relies on receptor internalization, clathrin-coated pits, and the GTPase dynamin, which facilitates the formation of endocytic vesicles from the plasma membrane.
Unlike neurons, microglia have the ability to internalize and degrade tau through endocytosis, potentially reducing its pathological spread. However, the specific endocytic mechanisms by which microglia process tau remain unclear. In collaboration with StressMarq Biosciences, a recent study by Falkon et al., published in Alzheimer’s & Dementia, has provided new insights into the precise endocytic pathways involved in microglial tau uptake and degradation. These findings highlight a potential therapeutic strategy—enhancing microglial clearance of tau to limit its propagation and reduce processes that contribute to neurodegenerative disease progression.
Mechanisms of endocytosis
To commence the study, researchers first needed to determine whether the two primary mechanisms of tau endocytosis in neurons—macropinocytosis and clathrin-mediated endocytosis—are also utilized by microglial cells. Scientists at The University of Texas Health Science Center, San Antonio subsequently investigated microglial endocytic pathways using well-characterized inhibitors and fluorescently tagged endocytic substrates.
Microglial tau uptake
After confirming that microglia utilize macropinocytosis and clathrin-mediated endocytosis for well-characterized substrates, researchers shifted their focus to understanding how tau is processed. To track tau uptake, tau proteins were labeled with AlexaFluor488, enabling visualization and quantification. The study examined different tau conformations, including StressMarq’s Tau-441 (2N4R) P301S Mutant Monomers (catalog #SPR-327) and Pre-formed Fibrils (catalog #SPR-329).
Importantly, the fluorescent tagging did not alter the size distribution of any tau conformation, ensuring that the results reflected natural tau processing. Researchers then tested a range of chemical inhibitors targeting endocytic pathways in the presence of tau monomers or fibrils, using microscopy-based quantification. The dynamin inhibitor Dyngo 4a significantly reduced the uptake of both tau monomers and fibrils. These findings were further validated using flow cytometry, confirming that tau endocytosis in microglia is dependent on dynamin, a GTPase enzyme essential for separating endocytic vesicles from the plasma membrane.
Differential receptor-mediated endocytosis
Receptor-mediated endocytosis is initiated by canonical receptors, which play a crucial role in tau uptake. In neurons, tau internalization via macropinocytosis is mediated by heparan sulfate proteoglycan (HSPG) on the cell surface. HSPG can also collaborate with low-density lipoprotein receptor-related protein-1 (LRP1) to facilitate tau uptake through clathrin-mediated endocytosis. Similar mechanisms have been observed in astrocytes—specialized glial cells that support neuronal survival. However, the role of HSPG in microglia remains less understood, prompting researchers to investigate its function in these immune cells.
Given that HSPG can act as a receptor for tau uptake, researchers used heparin, a structurally similar molecule, as a competitive antagonist to block HSPG-tau interactions. Their findings revealed that heparin treatment significantly reduced the uptake of tau fibrils in microglia but had no effect on tau monomers. Meanwhile, treatment with Rap, an LRP1 antagonist, had no impact on fibrillar tau uptake and only a modest but significant effect on monomeric tau uptake.
These findings were initially obtained using the BV2 mouse microglial cell line and further validated in primary murine microglia, which better represent physiological conditions. Consistent with the results from BV2 cells, tau uptake in primary microglia was found to be a dynamin-dependent process. Moreover, heparin effectively reduced HSPG-mediated uptake of tau fibrils without altering monomeric tau internalization.
Utilizing StressMarq products to decipher tau endocytic processes in microglia
Summary
The misfolding and aggregation of tau play a crucial role in the progression of tau pathology in Alzheimer’s disease. Pathogenic tau spreads between cells, where aggregated tau seeds trigger the misfolding of physiological tau, further driving disease progression. Microglia, the brain’s immune cells, play a dual role in neurodegeneration: while they can release neurotoxic tau seeds, they also exhibit neuroprotective functions by internalizing and degrading tau, thereby reducing its aggregation. However, until now, the mechanisms underlying tau endocytosis in microglia have remained largely unexplored.
The study by Falkon et al. provides the first comprehensive insights into the endocytic pathways involved in tau internalization in microglia, as well as the potential for pharmacological inhibition of these processes. Their findings confirm that microglial tau uptake is dependent on dynamin-mediated endocytosis, including clathrin-mediated endocytosis and macropinocytosis. Moreover, they demonstrate that antagonists targeting receptor-mediated tau uptake can specifically inhibit the internalization of pathogenic tau fibrils, offering a potential strategy to limit tau propagation.
By elucidating the mechanisms that regulate microglial tau internalization, this research paves the way for targeted therapies that enhance microglial clearance of tau, ultimately reducing intracellular tau spread and mitigating neurodegenerative progression.
Related StressMarq products
StressMarq Biosciences is committed to advancing neurodegenerative disease research through the development of innovative tools and collaborations. CEO Ariel Louwrier and former lead R&D scientist Jacob McPhail partnered with researchers from The University of Texas Health Science Center, San Antonio, USA, to contribute to this study.
To facilitate Alzheimer’s disease research, Tau dGAE (AA 297-391) (catalog #SPR-502) AD-mimic Pre-formed Fibrils replicate the structure and function of pathological tau fibrils commonly found in neurofibrillary tangles. Additionally, Tau-441 (2N4R) P301S Pre-formed Fibrils (CHO-expressed, N-glycosylated) (catalog #SPR-516) closely model tau aggregates observed in human tauopathies, making them ideal for disease modeling.
For more details on our latest scientific publications and our cutting-edge range of tau, alpha synuclein, and amyloid beta proteins, please visit our website.
References
- Microglia internalize tau monomers and fibrils using distinct receptors but similar mechanisms. Falkon, K. F, et al. Alzheimer’s & Dementia. November 2024.

Leave a Reply