Exploring Tau Oligomers in Neurodegenerative Diseases
As of March 2024, the World Health Organization (WHO) estimates that approximately 50 million people worldwide are affected by neurodegenerative diseases. While the factors that influence the development of these diseases are varied and complex, Alzheimer’s disease (AD), Parkinson’s disease (PD) and frontotemporal dementia (FTD) are characterized by progressive neuronal loss and corresponding cognitive and functional decline.
In the healthy human brain, the microtubule-associated tau protein plays a critical role in the stabilization of the neuronal cytoskeleton. However, in neurodegenerative diseases, tau abnormally accumulates into toxic aggregates. Over the years, research has increasingly focused on oligomeric tau. It is thought that this form of tau demonstrates highly toxic properties, and may be the predominant enabling force behind several neurodegenerative disease pathologies. Understanding the role of tau oligomers in these disease mechanisms is crucial for developing potential therapeutic strategies.
Tau Oligomers
Under normal physiological conditions, the tau protein exists in a highly soluble form and is primarily concentrated in the cerebral cortex. In this healthy state, tau stabilizes cytoskeletal microtubules and facilitates the axonal transport of nutrients and molecules throughout the cell.
Modifications to the tau protein, such as hyperphosphorylation, disrupt its affinity for microtubules and affect its solubility. The result is the formation of tau aggregates that are toxic to neurons and easily spread throughout the brain. These aggregates include tau oligomers and neurofibrillary tangles (NFTs) and are observed in the pathological progression of several neurodegenerative diseases.
Tau oligomers are intermediate, soluble assemblies formed during the aggregation process. Unlike NFTs, which are large and insoluble, tau oligomers are smaller and more diffusible, enabling them to spread throughout the brain. Their unique structural properties enhance their neurotoxicity. While NFTs were long believed to be the primary pathological hallmark of tau-related neurodegeneration, increasing evidence suggests that tau oligomers may play a more direct role in neuronal dysfunction and disease progression.
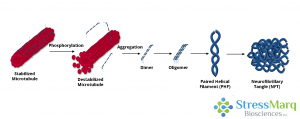
Tau dissociates from microtubules, leading to their destabilization. It then aggregates into oligomers, paired helical filaments, and ultimately neurofibrillary tangles.
Mechanisms of Toxicity
Synaptic Dysfunction and Neurotoxicity: Oligomeric tau is thought to disrupt synaptic function in multiple ways. One theory is that this form of tau impairs synaptic plasticity, which is essential for learning and memory, by interfering with key signaling pathways. For instance, tau oligomers have been shown to disrupt post-synaptic receptors such as NMDA and AMPA, leading to dysregulated calcium influx and excitotoxicity. They can also impair mitochondrial function, reducing energy supply to neurons and exacerbating synaptic loss.
Microtubule Destabilization: Under healthy physiological conditions, the tau protein plays a significant role in the stabilization of microtubules – cytoskeletal structures that are vital for intracellular transport in eukaryotic cells. In the process of oligomerization, tau dissociates from microtubules, resulting in destabilization and impairment of axonal transport. This disruption hinders the movement of essential cellular components such as organelles and signaling molecules, further enabling neuronal degeneration.
Cell-to-Cell Propagation: It has been established that tau oligomers also exhibit prion-like behavior, meaning they can spread from cell to cell, propagating tau pathology throughout the brain. This process may occur through exosomes or through direct release into the extracellular space, where the toxic species are taken up by neighboring neurons. This mechanism is believed to drive the characteristic progression of tau pathology in neurodegenerative diseases such as AD, where specific brain regions are affected sequentially over time.
Inflammatory Response: The presence of oligomeric tau species may potentially activate microglia, the brain’s resident immune cells, triggering a chronic inflammatory response. Enhanced neuroinflammation may exacerbate neuronal damage, creating a detrimental cycle that accelerates disease progression. Recent studies have shown that tau oligomers can also contribute to disintegration of the blood-brain barrier, further amplifying neuroinflammation and neuronal injury.
StressMarq’s Tau Oligomers
Given their central role in disease mechanisms, tau oligomers have quickly become a promising therapeutic target in several neurodegenerative diseases, with emerging data highlighting their significance. StressMarq’s new Tau-441 (2N4R) Wild-Type Oligomers (catalog# SPR-497) offer a valuable tool for researchers studying the role of tau oligomers in these diseases, providing a reliable and consistent model to explore tau aggregation and its toxic effects.
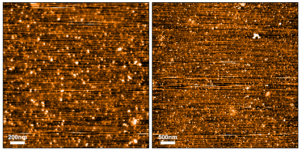
AFM images of Human Recombinant Tau-441 (2N4R) Wild-Type Oligomers (catalog# SPR-497).
New! Human Tau-441 (2N4R) Wild-Type Oligomers: StressMarq’s tau oligomers have been proven to exert significant dose-dependent toxicity in hippocampal neuron cultures. Expression in the Baculovirus/Sf9 system enables the generation of hyperphosphorylated tau, with up to 20 unique phosphorylation sites confirmed via mass spectrometry analysis. These wild-type oligomeric tau constructs are formed without the use of heparin or an anionic scaffold.
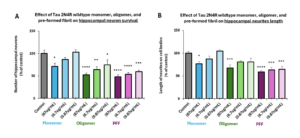
Effect of Human Recombinant Tau-441 (2N4R) Wild-Type (Baculovirus/Sf9) Monomers (catalog# SPR-496), Oligomers (catalog# SPR-497), and Pre-formed Fibrils (catalog# SPR-498) on hippocampal neuron survival and neurite length.
Specialized Protein Constructs for Neurodegenerative Disease Research
Tau oligomers are quickly emerging as crucial players in the pathogenesis of several neurodegenerative diseases. Their role in synaptic dysfunction, microtubule destabilization, and propagation of tau pathology makes them a compelling yet challenging therapeutic target. Worldwide research into the molecular mechanisms of tau oligomer toxicity and the development of tau-specific therapies includes strategies aimed at reducing tau oligomer formation, enhancing their clearance, or neutralizing their harmful impact.
Novel approaches such as small molecule inhibitors, immunotherapies, and gene therapies are currently under investigation. Additionally, monoclonal antibodies that specifically recognize tau oligomers and prevent their spread have yielded promising results in preclinical and early-stage clinical trials. Despite significant progress in the field, developing therapies that can selectively target toxic tau oligomers without disrupting normal tau function remains a complex challenge. The timing of intervention is also critical, as tau pathology is often well-established by the time clinical symptoms appear.
As an experienced manufacturer of fibrillar and oligomeric protein constructs for in vitro and in vivo research models, StressMarq is proud to make Tau Oligomers available to the research community. Our wide range of cutting-edge tau, alpha synuclein, and amyloid beta protein constructs for neurodegenerative disease research are available to researchers worldwide through our network of international distributors.
Rigorous Quality Control
StressMarq’s quality control testing for neuroproteins includes:
- Sedimentation assays to ensure that most of the monomer was converted to fibril
- EM/AFM imaging to verify fibril formation
- Thioflavin T assay to assess seeding capability of the fibrils
- SDS-PAGE to ensure protein purity
- Sterility check
- Endotoxin testing
Product Citations
Many of StressMarq’s monomeric, fibrillar and conjugated tau preparations have been cited in research publications. Certain products have also been validated in both in vitro and in vivo studies by various StressMarq collaborators.
References
- Uncovering the culprits of neurodegenerative disorders. Dimmer, O. Northwestern Medicine. 2024.
- NMDA and AMPA receptors at synapses: Novel targets for tau and α-synuclein proteinopathies. Italia, M. et al. Biomedicines. 2022.
- Role of tau protein in both physiological and pathological conditions. Avila, J. et al. Physiol Rev. 2004.


Leave a Reply