N-terminal Acetylated Alpha Synuclein in Parkinson’s Disease
Alpha synuclein is a 14 kDa protein that is abundant in the pre-synaptic terminals of neurons. It is best known for its association with Parkinson’s disease and other synucleinopathies, where it aggregates to form inclusion structures called Lewy bodies – the classic pathological hallmark of these conditions.
Although alpha synuclein has long been regarded as an intrinsically disordered protein, it was found in 2009 to adopt an alpha-helical conformation upon binding to lipid membranes, indicating a possible physiological role for the alpha-helical state in synaptic vesicle cycling1,2. In contrast, the beta-sheet-rich conformation has been closely linked to disease pathogenesis, demonstrating both neuronal toxicity and the ability to seed alpha synuclein aggregates in vivo3. This distinction between the two conformations is important, not least because enhanced alpha-helical folding can increase resistance to aggregation. Targeting the mechanisms that underlie alpha synuclein conformation promises to be an effective therapeutic strategy for a broad range of neurodegenerative disorders.
Alpha synuclein post-translational modifications
Various post-translational modifications (PTMs) have been proposed to modulate the function of alpha synuclein, with phosphorylation, truncation, and ubiquitination being among the most widely studied to date4. These PTMs exert their effects in very different ways.
For example, while mono- and di-ubiquitinated forms of alpha synuclein are found in Lewy bodies, polyubiquitinated alpha synuclein is instead targeted for proteasomal degradation; and while approximately 90% of the alpha synuclein in Lewy bodies is phosphorylated at serine 129, only around 4% of total alpha synuclein in the normal brain features this modification 5,6. More recently, N-terminal acetylation of alpha synuclein has been investigated for its therapeutic utility, largely due to the protective function this particular PTM performs.
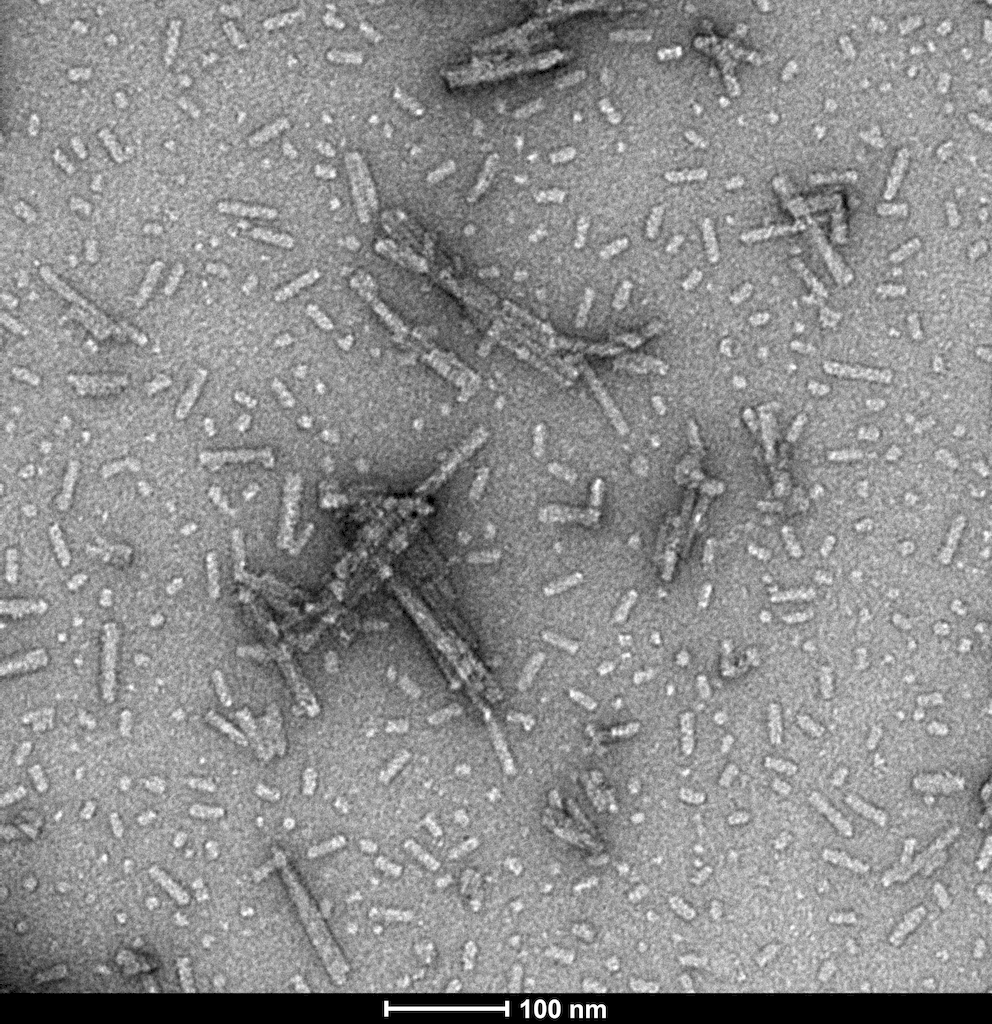
TEM of N-terminal acetylated alpha synuclein PFFs (catalog# SPR-332)
How does N-terminal acetylation affect alpha synuclein aggregation?
Although numerous reports have indicated that all alpha synuclein is post-translationally modified in vivo by the addition of an acetyl group at the N-terminus, many in vitro experiments have relied on recombinant forms of the protein lacking this modification. However, studies using N-acetylated alpha synuclein have yielded interesting findings.
Where small vesicles of negatively charged lipids were used as model membranes, it was found that N-terminal acetylation of alpha synuclein enhanced membrane-induced alpha-helical folding and resulted in complete resistance of the folded protein to fibrillar aggregation, a finding which was not replicated by the non-acetylated protein2. Additionally, mutant forms of alpha synuclein have shown the N-terminus to determine its neuronal toxicity, most likely through its acetylation. While other studies have revealed N-terminally acetylated alpha synuclein to have significantly reduced sensitivity to thioflavin T, a reagent commonly used to seed aggregation from alpha synuclein monomers7,8. In combination, these findings highlight the value of using N-acetylated alpha synuclein for research aimed at exploiting this post-translational modification for therapeutic purposes.
Supporting the study of N-terminal alpha synuclein acetylation
StressMarq offers a comprehensive portfolio of reagents for researchers studying Parkinson’s disease and other synucleinopathies and has recently launched N-Terminal Acetylated Alpha Synuclein Monomers (catalog# SPR-331) and N-Terminal Acetylated Alpha Synuclein Pre-Formed Fibrils (catalog# SPR-332). These demonstrate increased fluorescence, indicative of beta-sheet formation, when combined in a Thioflavin T seeding assay.
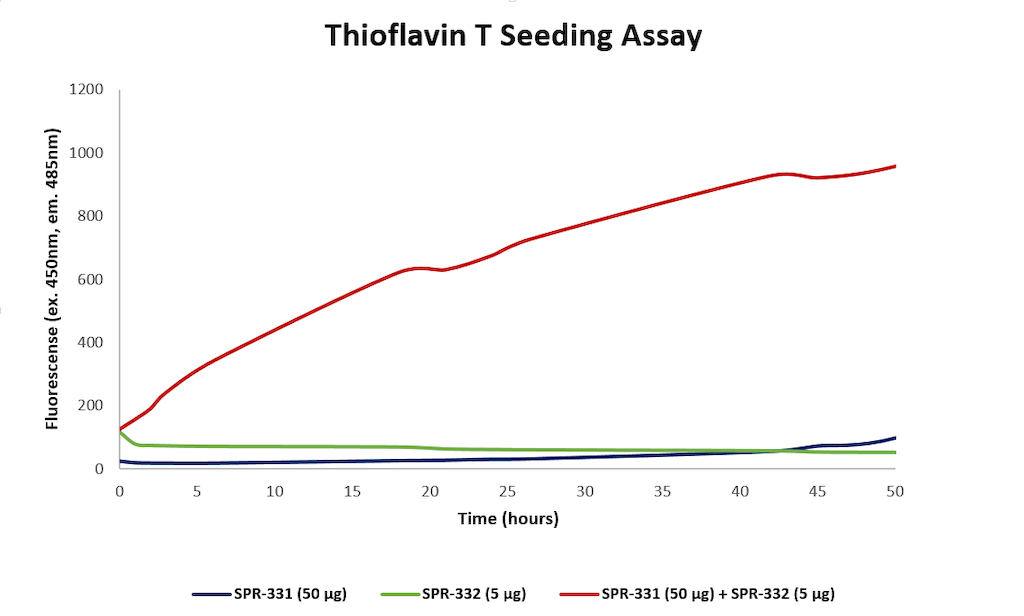
Thioflavin T assay for N-terminal acetylated alpha synuclein monomers (catalog# SPR-331) and pre-formed fibrils (catalog# SPR-332). Increased fluorescence, indicative of beta-sheet formation, was seen when 50 ug N-acetylated monomer and 5 ug N-acetylated fibril were combined, compared to monomer or fibril alone.
References
- Alpha-synuclein binds large unilamellar vesicles as an extended helix, Trexler AJ and Rhoades E, Biochemistry. 2009 Mar 24;48(11):2304-6
- N-alpha-acetylation of α-synuclein increases its helical folding propensity, GM1 binding specificity and resistance to aggregation, Bartels T et al, PLoS One 2014 Jul 30; 9(7)
- Endogenous alpha-synuclein monomers, oligomers and resulting pathology: let’s talk about the lipids in the room, Killinger BA et al, NPJ Parkinsons Dis. 2019 Nov 12;5: 23
- Role of post-translational modifications in modulating the structure, function and toxicity of alpha-synuclein: implications for Parkinson’s disease pathogenesis and therapies, Oueslati A et al, Prog Brain Res. 2010;183:115-45
- Ubiquitination of α-synuclein by Siah-1 promotes α-synuclein aggregation and apoptotic cell death, Lee JT et al, Hum Mol Genet. 2008 Mar 15;17(6):906-17
- The role of Ser129 phosphorylation of α-synuclein in neurodegeneration of Parkinson’s disease: a review of in vivo models, Sato H et al, Rev Neurosci. 2013;24(2):115-23
- N-terminal acetylation mutants affect alpha-synuclein stability, protein levels and neuronal toxicity, Vinueza-Gavilanes R et al, Neurobiol Dis. 2020 Apr;137:104781
- N-Terminal Acetylation Affects α-Synuclein Fibril Polymorphism, Watson MD and Lee JC, Biochemistry 2019, 58, 35, 3630–3633
- The influence of N-terminal acetylation on micelle-induced conformational changes and aggregation of α-Synuclein, Ruzafa D et al, PLoS ONE 2017; 12(5)


is native form of alphasynuclein has acteylated group at N terminal and recombinant alphasynuclein?
Thank you for your interest in our blog post. One of our technical staff will follow up with you directly to answer your question.