The Neuroprotective Effects of Cornel Iridoid Glycoside in Alzheimer’s Disease
Alzheimer’s disease (AD) is considered one of the most devastating neurodegenerative disorders worldwide as millions of people suffer from this severe form of dementia. One of the main AD hallmarks is the presence of neurofibrillary tangles (NFTs) which are composed of abnormally hyperphosphorylated tau protein. Tau protein and its post-translational modifications are implicated in the disease progression and can be targeted as a potential strategy for Alzheimer’s treatment. There are multiple factors that contribute to disease progression and there is currently no cure—only medication that can temporarily ease symptoms. Medicinal plants have been widely used in traditional medicine for their neuroprotective properties1 and are currently being investigated as potential Alzheimer’s treatments.
Pharmacological Activities of Cornus officinalis
Cornus officinalis Sieb. et Zucc. (CO), also called Asiatic dogwood, is a shrub native to China, Korea and Japan. The fruits (Fructus corni) have been widely used in traditional Chinese medicine for over 2000 years to nourish the liver and kidneys and treat chronic inflammation, diabetes and oxidative stress2. Iridoid glycosides, Morroniside and Loganin, also called Cornel Iridoid Glycoside (CIG), are secondary metabolites found in Fructus corni3. Recent studies demonstrated that CIG also exhibits neuroprotective effects as it improves memory, promotes neurogenesis and reduces tau hyperphosphorylation in Alzheimer’s disease3,4,5.

Cornus officinalis red fruits (Alpsdake, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons)
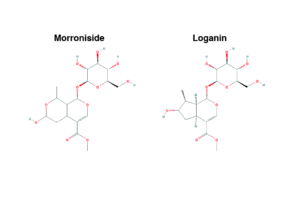
Chemical structure of cornel iridoid glycoside represented by morroniside and loganin (https://pubchem.ncbi.nlm.nih.gov/)
CIG as a potential treatment of Alzheimer’s Disease
A research group from Xuanwu Hospital of Capital Medical University in China, used our 2N4R tau monomers (SPR-327) and preformed fibrils (SPR-329) to investigate the impact of Cornel Iridoid Glycoside on tau aggregation. The study was published in the Current Medical Science journal and demonstrated that the iridoid extract from Cornus officinalis can be a potential therapeutic strategy for AD treatment. Specifically, the researchers investigated the effect of CIG on tau PTMs and synaptic abnormalities in PS19 transgenic mice.
The study demonstrated that:
CIG decreased tau hyperphosphorylation and neurofibrillary tangles but had no effect on C-terminal truncation of tau.
CIG (100 and 200 mg/kg) was administered daily to 10 month-old PS19 transgenic mice for 1 month. Mouse brains showed significant reduction of tau hyperphosphorylation levels measured by Western Blot.
CIG inhibited tau aggregation in a cell-free assay and disassembled tau aggregates.
StressMarq’s Tau441 (2N4R), P301S mutant monomers and preformed fibrils (PFFs) were used in this study to show how CIG affects tau aggregation in a cell-free system. Thioflavin T analysis demonstrated that incubation of tau fibrils with CIG for 1 h decreased fibril levels as well as tau oligomers.
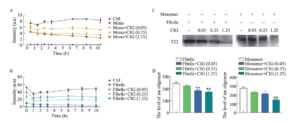
Effects of CIG on tau aggregation
A. ThT analysis of heparin-induced aggregation in physiologic tau. Monomer tau was induced using sodium heparin and thioflavin T and CIG (mg/kg) were added for 10h at 370C. B. ThT analysis of tau fibril. Fibril tau was incubated with thioflavin T and CIG for 10h at 370C. C,D. Representative Western Blots (C) and semiquantitative analysis (D) of the tau oligomer (T22). **P<0.01 vs. P301S group (Yang, Cc., et al. 2020 Cornel Iridoid Glycoside Regulates Modification of Tau and Alleviates Synaptic Abnormalities in Aged P301S Mice. CURR MED SCI 40, 1040–1046)

TEM of recombinant Tau-441 (2N4R), P301S mutant Pre-formed Fibrils (catalog# SPR-329). Fibrils were sonicated and stained with uranyl acetate.
Morroniside and Loganin interacted with tau
This interaction between the two CIG components and tau may reduce the formation of toxic tau oligomers.
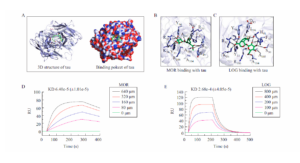
Interaction between MOR or LOG and Tau
A.The 3D structure and binding pocket of the tau protein. B-E. Interaction between MOR or LOG and tau using Autodock (B,C) and SPR (D,E). (Yang, Cc., et al. 2020 Cornel Iridoid Glycoside Regulates Modification of Tau and Alleviates Synaptic Abnormalities in Aged P301S Mice. CURR MED SCI 40, 1040–1046)
CIG improves synaptic abnormalities in PS19 mice.
Synaptic loss is one of the AD features leading to cognitive decline and several presynaptic proteins are affected. CIG administration, increased the expression of the presynaptic protein syntaxin1A, the postsynaptic PSD95 and the glutamate receptor subunits NMDAR2B and GluR1/2. The results demonstrated that CIG can restore the synaptic protein levels and protect synaptic function.
REFERENCES
- Neuroprotective effects of medicinal plants. Uddin R. et al. 2013 EXCLI J. 12:541-545.
- Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. Huang J. et al. 2018 J Ethnopharmacol. 213:280-301.
- New Iridoid Derivatives from the Fruits of Cornus officinalisand Their Neuroprotective Activities. Ji LL. et al. 2019 Molecules.;24(3):625.
- Cornel iridoid glycoside attenuates tau hyperphosphorylation by inhibition of PP2A demethylation. Yang CC. et al. 2013 Evid Based Complement Alternat Med, 108486.
- Cornel Iridoid Glycoside Inhibits Tau Hyperphosphorylation via Regulating Cross-Talk Between GSK-3β and PP2A Signaling. Yang C. et al. 2018 Front Pharmacol, 9:682.


Leave a Reply