Novel Vaccine Strategies for Parkinson’s Disease
Parkinson’s disease (PD) is a progressive neurodegenerative disorder estimated to affect millions worldwide. Hallmark motor symptoms, including tremors, stiffness and slowness of movement, were originally described more than 200 years ago by James Parkinson. Today, enhanced research efforts have allowed scientists to elucidate numerous motor and non-motor symotoms of PD. It has been established that a driving force behind the development of these symptoms is the degeneration, and subsequent loss, of dopaminergic neurons as a result of disease progression. The neuroinflammatory accumulation of alpha synuclein plays a key role in dopamine loss through the impairment of mitochondrial function.
While the precise trigger for the disease remains unknown, current research suggests that the stimulating factors are diverse and multi-faceted. The available therapies targeting PD only aim to alleviate its symptoms, with a cure or disease-modifying therapy still under investigation. One approach to preventing Parkinson’s disease that has been of particular interest in recent years is the development of vaccines that stimulate the body to produce its own antibodies against alpha synuclein. Scientific findings by Park et al. published in Vaccines depict the successful design of an alpha synuclein-specific epitope vaccine.
This novel vaccine has shown promising results in reducing the formation of neurotoxic alpha synuclein aggregates and, importantly, in alleviating the reduction of dopamine-related markers. Moreoever, vaccination was also observed to decrease neuroinflammation in the brain by reducing microglial activation and the subsequent release of pro-inflammatory cytokines. The successful development of a vaccine targeting PD pathology represents a potentially effective approach to preventing the development of Parkinson’s disease and provides an optimisitic outlook for the future of neurodegenerative disease research.
Modeling Parkinson’s pathology
Researchers from Gyeongsang National University in South Korea undertook a significant project focussed on the design of a non-toxic alpha synuclein-based peptide-epitope vaccine, using an in silico modelling platform. This research holds great promise in preventing alpha synuclein aggregation, a critical process in Parkinson’s disease pathogenesis. The vaccine utilizes distinct peptide epitopes that can be recognized by B-cell surface receptors, leading to antibody generation during B-cell stimulation/maturation. For the vaccine, the researchers selected a 10 amino acid long peptide to induce B-cell activity. B-cell epitope analysis forecasted that the peptide would be effective, and the ToxinPred in silico research tool further postulated that the peptide epitope vaccine would be non-toxic.
Another essential consideration for epitope-based peptide vaccines is their ability to produce a strong immunoreactive response against the intended target, which can be challenging when employing a short peptide. To enhance the antigen’s size and immunogenicity, carrier proteins such as ovalbumin (OVA) and keyhole limpet hemocyanin (KLH) were added to the C-terminal region of the peptide. Subsequently, the peptide was further stabilized via N-terminal acetylation, in order to prevent proteolytic degradation. The effectiveness of the vaccine design was confirmed through in vivo experimental data, which depicted the efficient binding of the antigen to B cells and the consequent stimulation and production of alpha synuclein antibodies.
Effects of immunization on motor dysfunction
Parkinson’s disease models have been crucial to understanding the distinctive characteristics as well as the molecular mechanisms underlying disease initiation and progression. Administration of StressMarq’s Alpha Synuclein Pre-formed Fibrils (PFFs) (catalog #SPR-322) seeded the aggregation of endogenous alpha synuclein, subsequently inducing motor dysfunction — one of the most prominent symptoms of Parkinson’s disease.
Two behavioural tests were employed to ascertain whether the alpha synuclein vaccine was able to stabilize, or improve motor dysfunction — the open field test (OFT) and the rotarod performance test. The former is employed to monitor the locomotive ability of mice exposed to an open, unobstructed area, while the latter aims to measure fine balance and motor coordination skills. Interestingly, the data collected from both tests proved conclusively that prior immunization with the epitope vaccine in the alpha synuclein-induced Parkinson’s disease model led to significant amelioration of dyfunctional motor processes.
Measuring vaccine effectiveness
In this study, researchers investigated the efficacy of an alpha synuclein-based peptide-epitope vaccine in a Parkinson’s disease model induced with StressMarq’s Alpha Synuclein Pre-formed Fibrils (catalog #SPR-322). The proposed vaccine demonstrated the distinct ability to ameliorate Parkinson’s disease pathophysiology. Finally, the findings further highlight the utility and efficacy of StressMarq’s alpha synuclein protein constructs for use in neurodegenerative disease models.
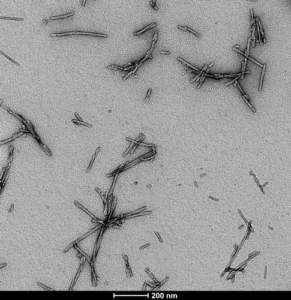
[Image from: StressMarq website.] Transmission electron microscopy (TEM) image of StressMarq’s Alpha Synuclein Pre-formed Fibrils (PFFs) (catalog #SPR-322)
Summary
Due to the increasingly aging global population, the prevalence of Parkinson’s disease has seen a dramatic increase in recent years. With current therapies targeted towards alleviating symptoms, but not disease etiology, vaccination strategies remain of vital interest as a tool to protect the population against the debilitating effects of Parkinson’s disease. The recent findings by Park et al. reveal that immunization with the non-toxic alpha synuclein peptide epitope is able to significantly reduce the build-up of alpha synuclein and the onset of motor and non-motor symptoms associated with PD. This represents a significant advancement in the establishment of strategies to prevent the development of Parkinson’s disease, and the field of neurogenerative disease research as a whole.
Related StressMarq products
StressMarq’s diverse range of alpha synuclein, tau and amyloid beta protein constructs provides researchers with an advantageous toolkit for the development of Parkinson’s research models. In addition to Human Alpha Synuclein Pre-formed Fibrils (catalog# SPR-322), StressMarq also manufactures Mouse Alpha Synuclein Monomers (catalog# SPR-323) & Pre-formed Fibrils (catalog# SPR-324). Visit our website for more information, including the latest scientific publications using our specialized proteins for neurodegenerative disease research.
References
- Immunization effects of a novel α-synuclein-based peptide epitope vaccine in Parkinson’s disease-associated pathology. Park, J. S., et al. Vaccines. 2023.
- Intrastriatal injection of pre-formed mouse α-synuclein fibrils into rats triggers α-synuclein pathology and bilateral nigrostriatal degeneration. Paumier, K. L., et al. Neurobiol Dis. 2023.


Leave a Reply