Properties
| Storage Buffer | PBS, 50% glycerol, 0.09% sodium azide *Storage buffer may change when conjugated |
| Storage Temperature | -20ºC, Conjugated antibodies should be stored according to the product label |
| Shipping Temperature | Blue Ice or 4ºC |
| Purification | Protein A purified |
| Clonality | Polyclonal |
| Specificity | Recognizes generic epitopes common to many amyloid fibrils and fibrillar oligomers, but not prefibrillar oligomers or natively folded proteins. Expected to detect in Mouse and Rat based on species homology. |
| Cite This Product | StressMarq Biosciences Cat# SPC-507, RRID: AB_10960639 |
| Certificate of Analysis | A 1:1000 dilution of SPC-507 was sufficient for detection of amyloid fibrils on PVDF membranes using transferred fibrils by colorimetric dot blot analysis using Goat anti-rabbit IgG:HRP as the secondary antibody. |
Biological Description
| Alternative Names | OC Antibody, Fibrils Antibody, Amyloid Oligomer aß Antibody, A11 Antibody, Amyloid beta A4 protein Antibody, ABPP Antibody, APPI Antibody, Alzheimer disease amyloid protein Antibody, Cerebral vascular amyloid peptide Antibody, PreA4 Antibody, Protease nexin-II Antibody, APP Antibody, A4 Antibody, AD Antibody |
| Research Areas | Alzheimer's Disease, Amyloid, Blood, Cardiovascular System, Cell Signaling, Neurodegeneration, Neuroscience |
| Cellular Localization | Membrane |
| Scientific Background | Amyloid monomeric proteins can sometimes oligomerize into destructive amyloid fibrils. Amyloidogenic conformations of non-disease related proteins can be created by partial protein misfolding or denaturation. Many degenerative diseases are known to be related to the accumulation of misfolded proteins as amyloid fibres (1, 2). These include the amyloid-β peptide plaques and tau neurofibrillary tangles in senile plaques of Alzheimer's symptomology, the deposition of α-synuclein in the Lewy bodies of Parkinson's disease, and accumulation of polyglutamine-containing aggregates in Huntington's disease (2, 3). |
| References |
1. Glabe C.G. (2004) Trends Biochem Sci. 29(10): 542-547. 2. Kayed R., et al. (2004) J Bio. Chem. 279: 46363-46366. 3. Kayed R., et al. (2003) Science. 300(5618): 486-489. |
Product Images
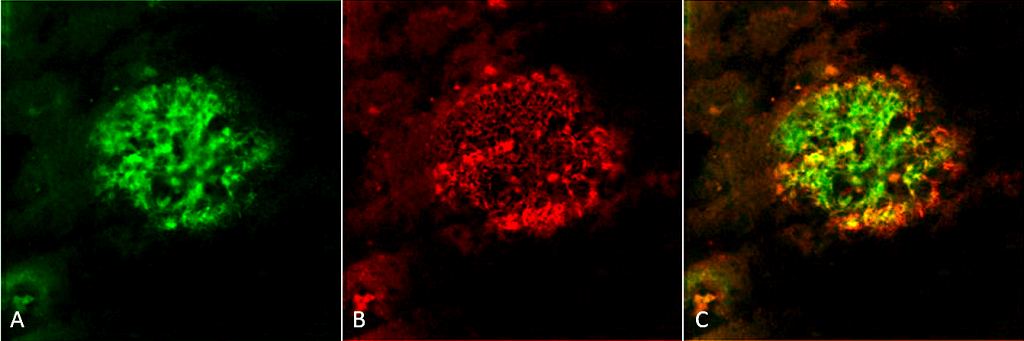
Immunohistochemistry analysis using Rabbit Anti-Amyloid Fibrils (OC) Polyclonal Antibody (SPC-507). Tissue: Alzheimer’s Disease brain. Species: Human. Fixation: Formalin fixed. Primary Antibody: Rabbit Anti-Amyloid Fibrils (OC) Polyclonal Antibody (SPC-507) at 1:5000. Secondary Antibody: Goat Anti-Rabbit ATTO 488 (green). Localization: Plaque. (A) Amyloid Fibril (OC) Antibody (SPC-507). (B) Amyloid Oligomer (A11) Antibody (SPC-506). (C) Composite. Courtesy of: Dr. Elizabeth Head, University of California, Irvine.
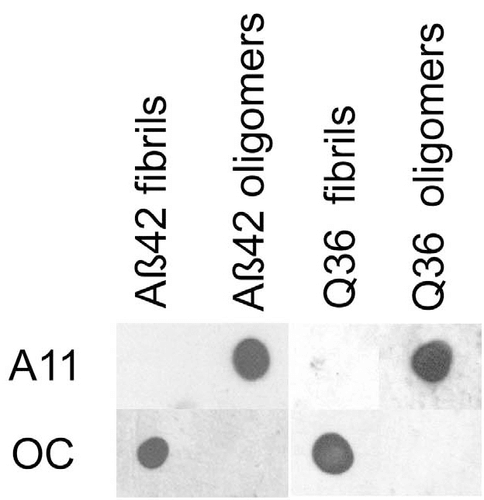
Dot blot analysis using Rabbit Anti-Amyloid Fibrils (OC) Polyclonal Antibody (SPC-507). Tissue: Abeta42 fibrils and prefibrillar oligomers. Species: Human. Primary Antibody: Rabbit Anti-Amyloid Fibrils (OC) Polyclonal Antibody (SPC-507) at 1:1000. Courtesy of: Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W., et al. (2003). Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489. doi: 10.1126/science.1079469.
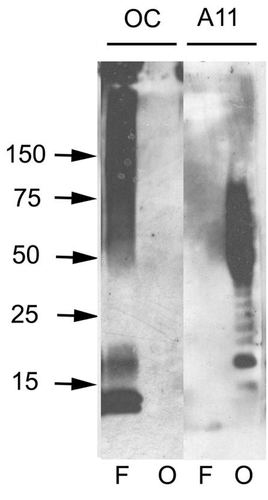
Western blot analysis of Human Abeta42 fibrils and prefibrillar oligomers showing detection of Amyloid Fibrils (OC) protein using Rabbit Anti-Amyloid Fibrils (OC) Polyclonal Antibody (SPC-507). Primary Antibody: Rabbit Anti-Amyloid Fibrils (OC) Polyclonal Antibody (SPC-507) at 1:1000. Courtesy of: Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W., et al. (2003). Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489. doi: 10.1126/science.1079469.
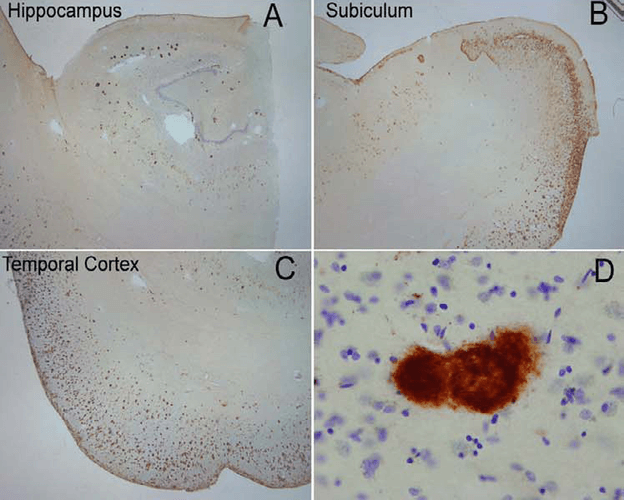
Immunohistochemistry analysis using Rabbit Anti-Amyloid Fibrils (OC) Polyclonal Antibody (SPC-507). Tissue: Alzheimer’s Disease brain. Species: Human. Primary Antibody: Rabbit Anti-Amyloid Fibrils (OC) Polyclonal Antibody (SPC-507) at 1:100. Extensive OC labeling was observed in the hippocampus (A), subiculum (B) and frontal cortex (C) in Alzheimer disease. A higher magnification image illustrates that OC positive deposits were dense and consisted of fine fibrillar material (D). Courtesy of: Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W., et al. (2003). Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489. doi: 10.1126/science.1079469.
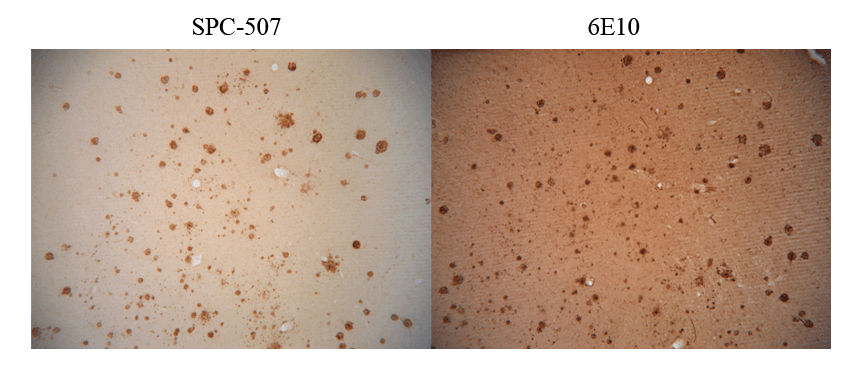
Immunohistochemistry analysis using Rabbit Anti-Amyloid Fibrils (OC) Polyclonal Antibody (SPC-507). Tissue: Alzheimer’s Disease brain. Species: Human. Primary Antibody: Rabbit Anti-Amyloid Fibrils (OC) Polyclonal Antibody (SPC-507) at 1:100. Showing no Amyloid Precursor Protein (APP) cross-reactivity (L), but when conducted with monoclonal 6E10 (R) shows considerable APP cross-reactivity. Courtesy of: Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W., et al. (2003). Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489. doi: 10.1126/science.1079469.
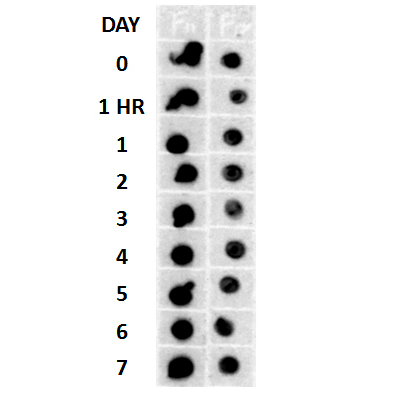
Dot blot analysis using Rabbit Anti-Amyloid Fibrils (OC) Polyclonal Antibody (SPC-507). Tissue: Cell lysates. Species: Human. Primary Antibody: Rabbit Anti-Amyloid Fibrils (OC) Polyclonal Antibody (SPC-507) at 1:500, 1:5000. Beta Amyloid HEPES-NaCl aggregation, showing 1:500 (L) and 1:5000 (R) time lapse dot blot.






StressMarq Biosciences :
Based on validation through cited publications.