Properties
| Storage Buffer | PBS pH7.2, 50% glycerol, 0.09% sodium azide *Storage buffer may change when conjugated |
| Storage Temperature | -20ºC, Conjugated antibodies should be stored according to the product label |
| Shipping Temperature | Blue Ice or 4ºC |
| Purification | Protein G Purified |
| Clonality | Monoclonal |
| Clone Number | 9G10 |
| Isotype | IgG2a |
| Specificity | Detects ~98kDa. Does not detect human HSP90, Grp74, or GrpE from E.coli. |
| Cite This Product | StressMarq Biosciences Cat# SMC-105, RRID: AB_2233345 |
| Certificate of Analysis | 0.5 µg/ml of SMC-105 was sufficient for detection of Grp94 in 20 µg of heat shocked HeLa cell lysate by colorimetric immunoblot analysis using Goat anti-rat IgG:HRP as the secondary antibody. |
Biological Description
| Alternative Names | HSP90B1 Antibody, GP96 Antibody, TRA1 Antibody, ECGP Antibody, 94 kDa glucose regulated protein antibody, 94 kDa glucose-regulated protein antibody, ECGP antibody, Endoplasmin antibody, Endothelial cell (HBMEC) glycoprotein antibody, ENPL_HUMAN antibody, Glucose regulated protein 94kDa antibody, gp96 antibody, gp96 homolog antibody, GRP 94 antibody, GRP-94 antibody, Heat shock protein 90 kDa beta member 1 antibody, heat shock protein 90kDa beta (Grp94), member 1 antibody, Heat shock protein, 90 kDa, beta, 1 antibody, HSP90B1 antibody, Stress inducible tumor rejection antigen GP96 antibody, TRA1 antibody, tumor rejection antigen (gp96) 1 antibody, Tumor rejection antigen 1 antibody, Tumor rejection antigen gp96 antibody, Tumor rejection antigen-1 (gp96) antibody |
| Research Areas | Cancer, Cancer Metabolism, Cell Signaling, ER Proteins, Heat Shock, Hypoxia, Metabolism, Metabolism processes, Organelle Markers, Protein Trafficking, Response to Hypoxia, Tags and Cell Markers |
| Cellular Localization | Endoplasmic Reticulum, Endoplasmic reticulum lumen, Melanosome |
| Accession Number | NP_003290.1 |
| Gene ID | 7184 |
| Swiss Prot | P14625 |
| Scientific Background | Grp94 (glucose regulated protein 94, gp96) is a constitutively expressed endoplasmic reticulum (ER) lumenal protein that is up-regulated in response to cellular stress such as heat shock, oxidative stress or glucose depletion. Grp94 is thought to play a role in protein translocation to the ER, in their subsequent folding and assembly, and in regulating protein secretion (1). Grp94 also plays a role in antigen presentation by accessing the endogenous pathway and eliciting specific CTL responses to chaperone bound peptides via MHC class I pathway (2). Grp94 is a member of the HSP90 family of stress proteins and shares sequence homology with its cytosolic equivalent, HSP90 (3). Both HSP90 and Grp94 are calcium binding proteins (4). Despite sharing 50% sequence homology over its N domains and complete conservation in its ligand binding domains with HSP90, Grp94 and HSP90 differ in their interactions with regulatory ligands as Grp94 has weak ATP binding and hydrolysis activity (5). Grp94 exists as a homodimer and the two subunits interact at two distinct intermolecular sites, C terminal dimerization domains and the N-terminal interacts with the middle domain of opposing subunits (6). Grp94 contains a carboxy terminal KDEL (Lys-Asp-Glu-Leu) sequence which is believed to aid in its retention in the ER (7). |
| References |
1. Rudolph R.W., and Bedows E. (1997) J Biol Chem 272: 3125-3128. 2. Srivastava P.K., et al. (1994) Immunogenetics. 39(2):93-98. 3. Mazzarella R.A., and Green M. (1987) J Biol Chem 262:8875-8883. 4. Kang, H.S. and Welch W.J. (1991) J Biol Chem 266(9):5643-5649. 5. Soldano K.L., et al. (2003) J Biol Chem 278(48): 48330-48338. 6. Chu F., et al. (2006) Protein Sci 15(6): 1260-1269. 7. Peter F., et al., (1992) J Biol Chem 267: 10631-10637. 8. Allen S. et al. (2000) Blood 96(2): 560-568. 9. Sato K et al. (2001) Blood 98(6): 1852-1857. 10. Yun S.-W. et al (2000) Brain Research Bulletin.52(5):371-378. 11. Choukhi A., et al. (1998) J. Virol. 72: 3851-3858. 12. Hoshino T., et al. (1998) Blood 91(11): 4379-4386. 13. Riera M. et al. (1999) Mol. Cell Biochem. 191: 97-104. 14. Gusarova V., et al. (2001) J. Biol. Chem. 276(27): 24891-24900. |
Product Images
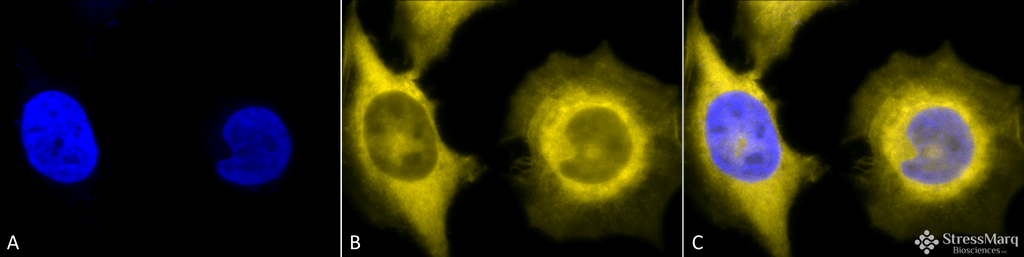
Immunocytochemistry/Immunofluorescence analysis using Rat Anti-GRP94 Monoclonal Antibody, Clone 9G10 (SMC-105). Tissue: Heat Shocked cervical cancer cells (HeLa). Species: Human. Fixation: 2% Formaldehyde for 20 min at RT. Primary Antibody: Rat Anti-GRP94 Monoclonal Antibody (SMC-105) at 1:100 for 12 hours at 4°C. Secondary Antibody: R-PE Goat Anti-Rat (yellow) at 1:200 for 2 hours at RT. Counterstain: DAPI (blue) nuclear stain at 1:40000 for 2 hours at RT. Localization: Endoplasmic reticulum lumen. Melanosome. Magnification: 100x. (A) DAPI (blue) nuclear stain. (B) Anti-GRP94 Antibody. (C) Composite. Heat Shocked at 42°C for 1h.
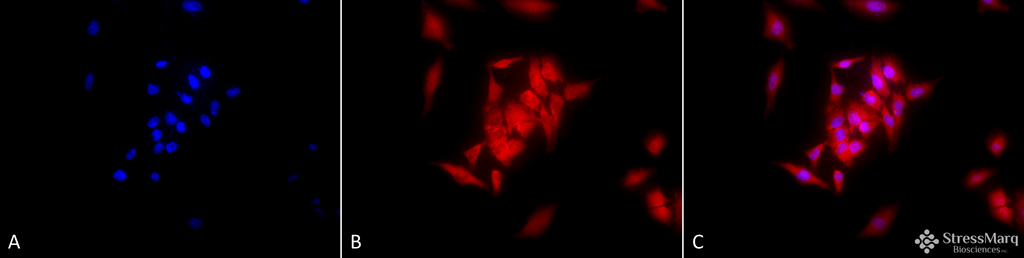
Immunocytochemistry/Immunofluorescence analysis using Rat Anti-GRP94 Monoclonal Antibody, Clone 9G10 (SMC-105). Tissue: Heat Shocked cervical cancer cells (HeLa). Species: Human. Fixation: 2% Formaldehyde for 20 min at RT. Primary Antibody: Rat Anti-GRP94 Monoclonal Antibody (SMC-105) at 1:100 for 12 hours at 4°C. Secondary Antibody: APC Goat Anti-Rat (red) at 1:200 for 2 hours at RT. Counterstain: DAPI (blue) nuclear stain at 1:40000 for 2 hours at RT. Localization: Endoplasmic reticulum lumen. Melanosome. Magnification: 20x. (A) DAPI (blue) nuclear stain. (B) Anti-GRP94 Antibody. (C) Composite. Heat Shocked at 42°C for 1h.

![Rat Anti-GRP94 Antibody [9G10] used in Immunocytochemistry/Immunofluorescence (ICC/IF) on Human Heat Shocked cervical cancer cells (HeLa) (SMC-105)](https://www.stressmarq.com/wp-content/uploads/SMC-105_GRP94_Antibody_9G10_ICC-IF_Human_Heat-Shocked-HeLa-Cells_20x_Composite-100x100.png)
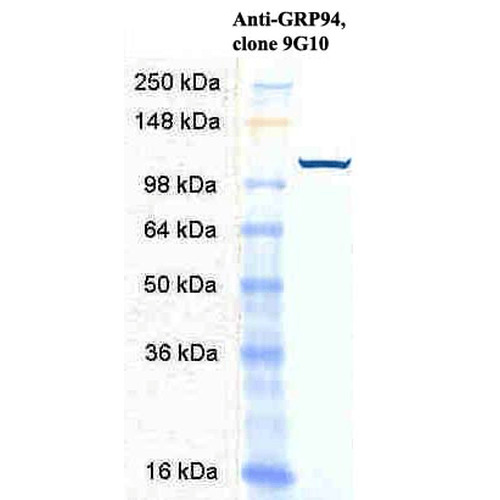
![Rat Anti-GRP94 Antibody [9G10] used in Western Blot (WB) on Human Cervical cancer cell line (HeLa) lysate (SMC-105)](https://www.stressmarq.com/wp-content/uploads/SMC-105_GRP94_Antibody_9G10_WB_Human_HeLa-cell-lysates_1-100x100.png)




















StressMarq Biosciences :
Based on validation through cited publications.