Properties
| Storage Buffer | PBS, 50% glycerol, 0.09% sodium azide *Storage buffer may change when conjugated |
| Storage Temperature | -20ºC, Conjugated antibodies should be stored according to the product label |
| Shipping Temperature | Blue Ice or 4ºC |
| Purification | Protein G Purified |
| Clonality | Monoclonal |
| Clone Number | LK2 |
| Isotype | IgG1 |
| Specificity | Detects ~60kDa. |
| Cite This Product | StressMarq Biosciences Cat# SMC-111, RRID: AB_2121271 |
| Certificate of Analysis | 0.25 µg/ml of SMC-111 was sufficient for detection of HSP60 in 10 µg of heat shocked HeLa cell lysate by colorimetric immunoblot analysis using goat anti-mouse IgG as the secondary antibody. |
Biological Description
| Alternative Names | CPN60 Antibody, GROEL Antibody, HLD4 Antibody, HSP 60 Antibody, HSP65 Antibody, HSPD1 Antibody, HuCHA60 Antibody, SPG 13 Antibody |
| Research Areas | Cancer, Cell Signaling, Chaperone Proteins, Heat Shock, Organelle Markers, Protein Trafficking, Tags and Cell Markers |
| Cellular Localization | Mitochondrion, Mitochondrion Matrix |
| Accession Number | NP_002147.2 |
| Gene ID | 3329 |
| Swiss Prot | P10809 |
| Scientific Background | In both prokaryotic and eukaryotic cells, the misfolding and aggregation of proteins during biogenesis and under conditions of cellular stress are prevented by molecular chaperones. Members of the HSP60 family of heat shock proteins are some of the best characterized chaperones. HSP60, also known as Cpn60 or GroEl, is an abundant protein synthesized constitutively in the cell that is induced to a higher concentration after brief cell shock. It is present in many species and exhibits a remarkable sequence homology among various counterparts in bacteria, plants, and mammals with more than half of the residues identical between bacterial and mammalian HSP60 (1-3). Whereas mammalian HSP60 is localized within the mitochondria, plant HSP60, or otherwise known as Rubisco-binding protein, is located in plant chloroplasts. It has been indicated that these proteins carry out a very important biological function due to the fact that HSP60 is present in so many different species. The common characteristics of the HSP60s from the divergent species are i) high abundance, ii) induction with environmental stress such as heat shock, iii) homo-oligomeric structures of either 7 or 14 subunits which reversibly dissociate in the presence of Mg2+ and ATP, iv) ATPase activity and v) a role in folding and assembly of oligomeric protein structures (4). These similarities are supported by recent studies where the single-ring human mitochondrial homolog, HSP60 with its co-chaperonin, HSP10 were expressed in a E. coli strain, engineered so that the groE operon is under strict regulatory control. This study has demonstrated that expression of HSP60-HSP10 was able to carry out all essential in vivo functions of GroEL and its co-chaperonin, GroES (5). Another important function of HSP60 and HSP10 is their protective functions against infection and cellular stress. HSP60 has however been linked to a number of autoimmune diseases, as well as Alzheimer's, coronary artery diseases, MS, and diabetes (6-9). |
| References |
1. Hartl, F.U. (1996) Nature 381: 571-579. 2. Bukau, B. and Horwich, A.L. (1998) Cell 92: 351-366. 3. Hartl, F.U. and Hayer-Hartl, M. (2002) Science 295: 1852- 1858. 4. Jindal, S., et al. (1989) Molecular and Cellular Biology 9: 2279-2283. 5. La Verda, D., et al (1999) Infect Dis. Obstet. Gynecol. 7: 64-71. 6. Itoh, H. et al. (2002) Eur. J. Biochem. 269: 5931-5938. 7. Gupta, S. and Knowlton, A.A. J. Cell Mol Med. 9: 51-58. 8. Deocaris, C.C. et al. (2006) Cell Stress Chaperones 11: 116-128. 9. Lai, H.C. et al. (2007) Am. J. Physiol. Endocrinol. Metab. 292: E292-E297. 10. Gao, Y.L., et al (1995) J. of Immunology 154: 3548-3556. 11. Neuer, A., et al (1997) European Society for Human Reproduction and Embryology 12(5):925-929. 12. Bason, C., et al (2003) Lancet 362(9400): 1971-1977. |
Product Images

Immunohistochemistry analysis using Mouse Anti-Hsp60 Monoclonal Antibody, Clone LK-2 (SMC-111). Tissue: colon carcinoma. Species: Human. Fixation: Formalin. Primary Antibody: Mouse Anti-Hsp60 Monoclonal Antibody (SMC-111) at 1:100000 for 12 hours at 4°C. Secondary Antibody: Biotin Goat Anti-Mouse at 1:2000 for 1 hour at RT. Counterstain: Mayer Hematoxylin (purple/blue) nuclear stain at 200 µl for 2 minutes at RT. Localization: Inflammatory cells. Magnification: 40x. This image was produced using an amplifying IHC wash buffer. The antibody has therefore been diluted more than is recommended for other applications.
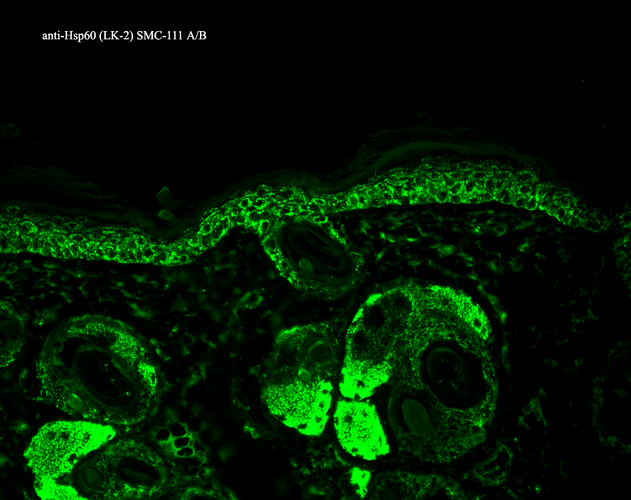
Immunohistochemistry analysis using Mouse Anti-Hsp60 Monoclonal Antibody, Clone LK-2 (SMC-111). Tissue: backskin. Species: Mouse. Fixation: Bouin’s Fixative and paraffin-embedded. Primary Antibody: Mouse Anti-Hsp60 Monoclonal Antibody (SMC-111) at 1:100 for 1 hour at RT. Secondary Antibody: FITC Goat Anti-Mouse (green) at 1:50 for 1 hour at RT. Localization: Cytoplasmic Staining.
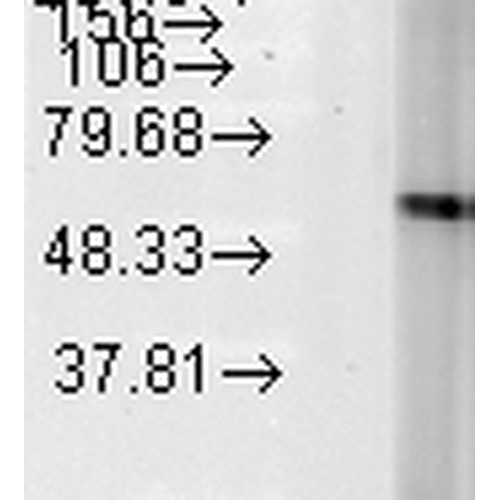
Western Blot analysis of Human Heat Shocked cervical cancer cell line (HeLa) lysate showing detection of Hsp60 protein using Mouse Anti-Hsp60 Monoclonal Antibody, Clone LK-2 (SMC-111). Load: 15 µg. Block: 1.5% BSA for 30 minutes at RT. Primary Antibody: Mouse Anti-Hsp60 Monoclonal Antibody (SMC-111) at 1:1000 for 2 hours at RT. Secondary Antibody: Sheep Anti-Mouse IgG: HRP for 1 hour at RT.


![Mouse Anti-Hsp60 Antibody [LK-2] used in Immunohistochemistry (IHC) on Mouse backskin (SMC-111)](https://www.stressmarq.com/wp-content/uploads/SMC-111_Hsp60_Antibody_LK-2_IHC_Mouse_backskin_1-100x100.png)
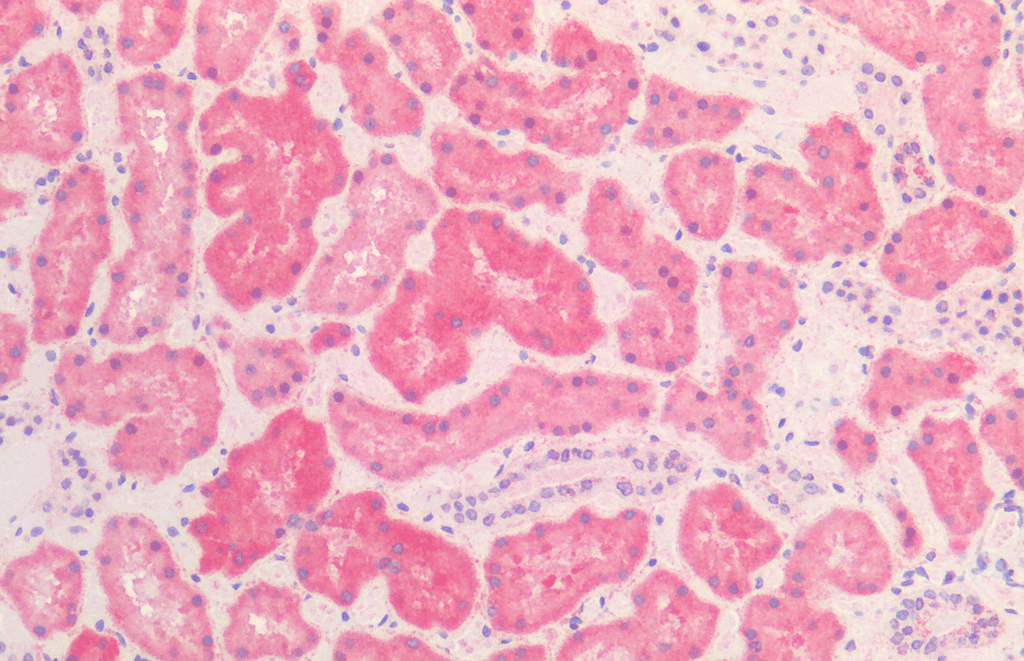
![Mouse Anti-HSP60 Antibody [LK2] used in Immunohistochemistry (IHC) on Human Kidney (SMC-111)](https://www.stressmarq.com/wp-content/uploads/SMC-111_HSP60_Antibody_LK2_IHC_Human_Kidney_1-100x100.png)
![Mouse Anti-Hsp60 Antibody [LK-2] used in Western Blot (WB) on Human Heat Shocked cervical cancer cell line (HeLa) lysate (SMC-111)](https://www.stressmarq.com/wp-content/uploads/SMC-111_Hsp60_Antibody_LK-2_WB_Human_Heat-Shocked-HeLa-cell-lysates_1-100x100.png)




















StressMarq Biosciences :
Based on validation through cited publications.