Properties
| Storage Buffer | PBS, 50% glycerol, 0.09% sodium azide *Storage buffer may change when conjugated |
| Storage Temperature | -20ºC, Conjugated antibodies should be stored according to the product label |
| Shipping Temperature | Blue Ice or 4ºC |
| Purification | Protein G Purified |
| Clonality | Monoclonal |
| Clone Number | 8A7 |
| Isotype | IgG1 Kappa |
| Specificity | Detects ~25kDa or ~27kDa. Recognizes HSP25 and HSP27, cross reacts with alpha B crystallin. |
| Cite This Product | HSP25/HSP27 Antibody (StressMarq Biosciences | Victoria, BC CANADA, Catalog# SMC-114, RRID: AB_2120775) |
| Certificate of Analysis | A 1:5000 dilution of SMC-114 was sufficient for detection of HSP27 in 20 µg of HeLa cell lysate by ECL immunoblot analysis. |
Biological Description
| Alternative Names | HSPB1, HSP27, HSP25, Heat shock protein beta-1, Heat shock 27 kDa protein, HSP28, MKBP, DMPK-binding protein |
| Research Areas | Actin, Actin Assembly, Atherosclerosis, Cancer, Cardiovascular System, Cell Signaling, Chaperone Proteins, Contractility, Cytoskeleton, Heart, Heat Shock, Microfilaments, Microtubules, Protein Trafficking, Tumor Biomarkers |
| Cellular Localization | Cytoplasm, Cytoskeleton, Nucleus, Spindle |
| Accession Number | NP_001532.1 |
| Gene ID | 3315 |
| Swiss Prot | P04792 |
| Scientific Background |
HSP25 (mouse) and its human homolog HSP27 are members of the small heat shock protein (sHSP) family, characterized by a conserved α-crystallin domain and a variable N-terminal region essential for oligomerization. These proteins form dynamic oligomers ranging from dimers to large multimers (8–40 monomers), with chaperone activity closely tied to their oligomeric state—larger assemblies exhibit potent anti-aggregation functions, while dimers are inactive. HSP27 is predominantly cytoplasmic under basal conditions but rapidly translocates to the nucleus in response to cellular stress, where it may stabilize nuclear structures and DNA. It is also rapidly phosphorylated in response to various stimuli, linking it to second messenger signaling pathways. Functionally, HSP27 acts as an ATP-independent molecular chaperone, preventing protein aggregation and stabilizing partially unfolded proteins, often in coordination with the HSP70 complex. In the nervous system, HSP27 plays a critical role in protecting neurons from proteotoxic stress, apoptosis, and oxidative damage—key features of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and ALS. It inhibits apoptotic signaling by blocking the cytochrome c/Apaf-1/procaspase-9 complex and may also influence cytoskeletal dynamics through interactions with actin and myosin. Upregulation of HSP27 correlates with increased phosphorylation and oligomer formation, suggesting a role in stress adaptation, cell differentiation, and potentially growth arrest. These properties make HSP27 a compelling target for therapeutic strategies aimed at enhancing neuronal resilience in neurodegenerative disease. |
| References |
1. Welch W.J. (1985) J Biol. Chem. 260: 3058-3062. 2. Kim K.K., Kim R., and Kim S. (1998) Nature 394(6693): 595-599. 3. Van Montfort R., Slingsby C., and Vierling E. (2001) Addv Protein Chem. 59: 105-56. 4. Ehrnsperger M., Graber S., Gaestel M. and Buchner J. (1997) EMBO J. 16: 221-229. 5. Ciocca D.R., Oesterreich S., Chamness G.C., McGuire W.L., and Fugua S.A. (1993) J Natl Cancer Inst. 85 (19): 1558-70. 6. Welsh M.J., Wu W., Parvinem M., and Gilmont R.R. (1996) Biol. Of Reprod. 55: 141-151. 7. Sarto C. Binnz P.A. and Mocarelli P. (2000) Electrophoresis. 21(6): 1218-26. 8. Arrigo A.P. (2005) J Cell Biochem. 94(2): 241-6. 9. Jia, Y. et al. (2001) J. Biol. Chem. 276(43):39911-39918. |
Product Images
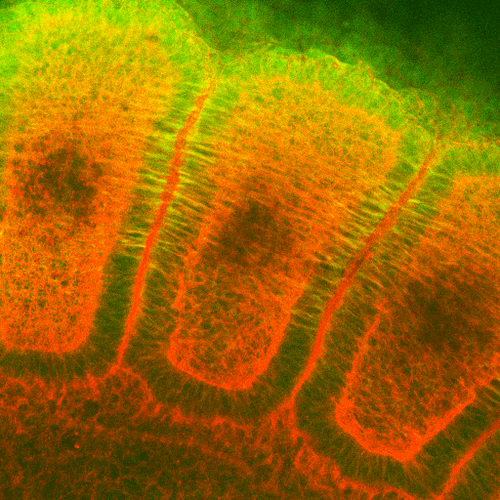
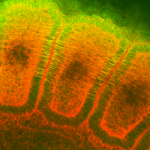
Immunohistochemistry analysis using Mouse Anti-Hsp27 Monoclonal Antibody, Clone 8A7 (SMC-114). Tissue: embryo somites. Species: Rat. Primary Antibody: Mouse Anti-Hsp27 Monoclonal Antibody (SMC-114) at 1:1000. Secondary Antibody: FITC Goat Anti-Mouse (green). Counterstain: Rhodamine-phalloidin labeled actin (red). Investigation at 11 days gestation. Courtesy of: Mike Welsh, Umich.

Immunohistochemistry analysis using Mouse Anti-Hsp27 Monoclonal Antibody, Clone 8A7 (SMC-114). Tissue: colon carcinoma. Species: Human. Fixation: Formalin. Primary Antibody: Mouse Anti-Hsp27 Monoclonal Antibody (SMC-114) at 1:5000 for 12 hours at 4°C. Secondary Antibody: Biotin Goat Anti-Mouse at 1:2000 for 1 hour at RT. Counterstain: Mayer Hematoxylin (purple/blue) nuclear stain at 200 µl for 2 minutes at RT. Localization: Inflammatory cells. Magnification: 40x.
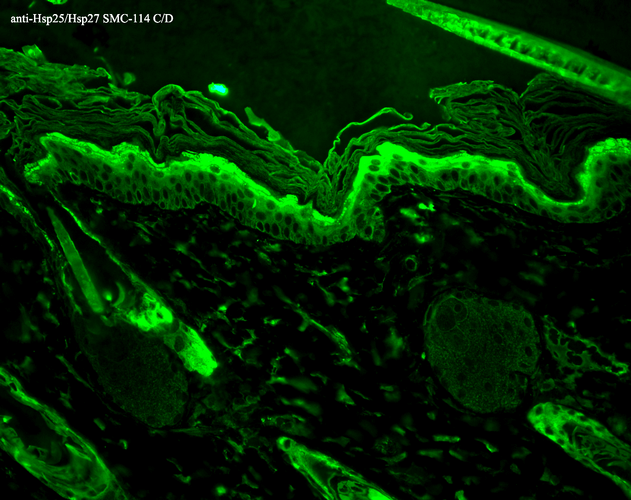
Immunohistochemistry analysis using Mouse Anti-Hsp27 Monoclonal Antibody, Clone 8A7 (SMC-114). Tissue: backskin. Species: Mouse. Fixation: Bouin’s Fixative and paraffin-embedded. Primary Antibody: Mouse Anti-Hsp27 Monoclonal Antibody (SMC-114) at 1:100 for 1 hour at RT. Secondary Antibody: FITC Goat Anti-Mouse (green) at 1:50 for 1 hour at RT. Localization: Epidermis.
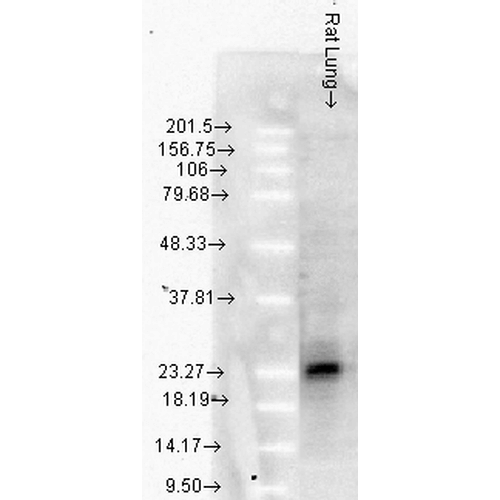
Western Blot analysis of Rat Lung tissue lysates showing detection of Hsp27 protein using Mouse Anti-Hsp27 Monoclonal Antibody, Clone 8A7 (SMC-114). Load: 15 µg. Block: 5% blocking solution. Primary Antibody: Mouse Anti-Hsp27 Monoclonal Antibody (SMC-114) at 1:1000 for 2 hours at RT. Secondary Antibody: Goat Anti-Mouse: HRP for 1 hour at RT.
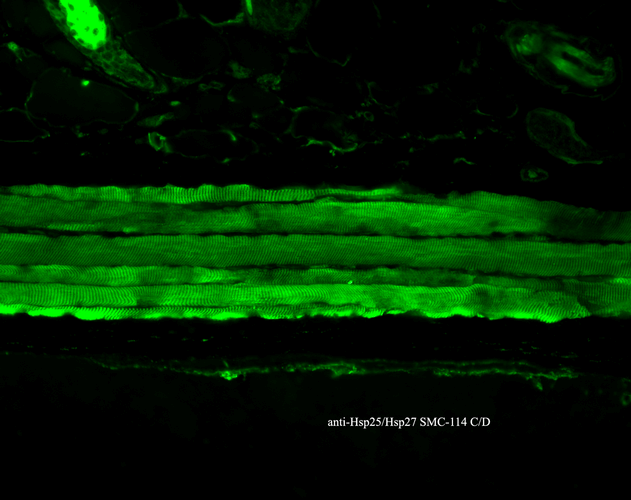
Immunohistochemistry analysis using Mouse Anti-Hsp27 Monoclonal Antibody, Clone 8A7 (SMC-114). Tissue: backskin. Species: Mouse. Fixation: Bouin’s Fixative and paraffin-embedded. Primary Antibody: Mouse Anti-Hsp27 Monoclonal Antibody (SMC-114) at 1:100 for 1 hour at RT. Secondary Antibody: FITC Goat Anti-Mouse (green) at 1:50 for 1 hour at RT. Localization: Epidermis.

![Mouse Anti-Hsp27 Antibody [8A7] used in Immunohistochemistry (IHC) on Human colon carcinoma (SMC-114)](https://www.stressmarq.com/wp-content/uploads/SMC-114_Hsp27_Antibody_8A7_IHC_Human_colon-carcinoma_40x_1-100x100.png)
![Mouse Anti-Hsp27 Antibody [8A7] used in Immunohistochemistry (IHC) on Mouse backskin (SMC-114)](https://www.stressmarq.com/wp-content/uploads/SMC-114_Hsp27_Antibody_8A7_IHC_Mouse_backskin_1-100x100.png)
![Mouse Anti-Hsp27 Antibody [8A7] used in Western Blot (WB) on Rat Lung tissue lysates (SMC-114)](https://www.stressmarq.com/wp-content/uploads/SMC-114_Hsp27_Antibody_8A7_WB_Rat_Lung-tissue-lysates_1-100x100.png)
![Mouse Anti-Hsp27 Antibody [8A7] used in Immunohistochemistry (IHC) on Mouse backskin (SMC-114)](https://www.stressmarq.com/wp-content/uploads/SMC-114_Hsp27_Antibody_8A7_IHC_Mouse_backskin_3-100x100.png)




















StressMarq Biosciences :
Based on validation through cited publications.