Properties
| Storage Buffer | 1X PBS pH 7.4 |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange & SEC purified |
| Cite This Product | Human Recombinant Alpha Synuclein E114C Mutant Pre-formed Fibrils: ATTO 488 (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SPR-518) |
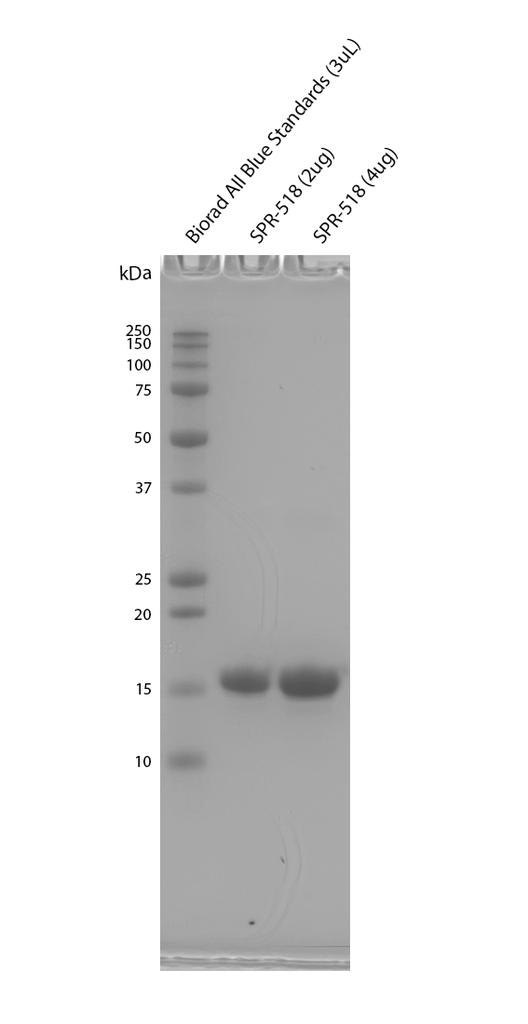
| Certificate of Analysis | Protein certified >95% pure on SDS-page and nanodrop analysis, endotoxin below 5 EU/mL at 2 mg/mL on starting monomer material. |
| Other Relevant Information | For best results, sonicate immediately prior to use. Refer to the Neurodegenerative Protein Handling Instructions on our website, or the product datasheet for further information. |
Biological Description
| Alternative Names | Alpha synuclein pre-formed fibril, Alpha-synuclein PFF, Alpha synuclein protein fibrils, Alpha-synuclein protein, Non-A beta component of AD amyloid protein, Non-A4 component of amyloid precursor protein, NACP protein, SNCA protein, NACP protein, PARK1 protein, SYN protein, Parkinson's disease familial 1 Protein |
| Research Areas | Alzheimer's Disease, Neurodegeneration, Neuroscience, Parkinson's Disease, Synuclein, Tangles & Tau, Multiple System Atrophy |
| Swiss Prot | P37840 |
| Scientific Background | The alpha-synuclein (aSyn) E114C mutation facilitates a single site-specific conjugation with ATTO-488 maleimide that avoids any hindrance on fibrilization or cell entry that may be conferred by non-specific lysine targeting conjugations. This conjugation is ideal due to internal position relative to C-terminal truncation sites, proximity to the NAC, and lack of interference with recruitment in vitro or in primary neurons (1, 2). Pre-formed fibrils (PFFs) generated with 5-25% fluorescently tagged E114C mutants have demonstrated a relative potency >80% compared to wild-type aSyn for inducing misfolding of endogenous aSyn, indicating no significant perturbation of seeding in living cells (1). Atto-488 is a useful tool for identifying cell entry, as the addition of Trypan Blue to cultures prior to imaging will quench fluorescence of extracellular Atto-488 conjugated aSyn (3). Our aSyn E114C-Atto-488 PFFs, which are formed from 10% fluorescently tagged E114C mutants and 90% wild-type monomers, are an excellent tool for studying cell entry and localization, with demonstrated entry into neurons after trypan blue quenching. |
| References |
1.,Haney et al. 2016. Comparison of strategies for non-perturbing labeling of α-synuclein to study amyloidogenesis. Organic & Biomolecular Chemistry. DOI: 10.1039/c5ob02329g 2.,Karpowicz et al. 2017. Selective imaging of internalized proteopathic a-synuclein seeds in primary neurons reveals mechanistic insight into transmission of synucleinopathies. JBC. DOI: 10.1074/jbc.M117.780296 3.,Pieri et al. 2016. Structural and functional properties of prefibrillar α-synuclein oligomers. Scientific Reports. DOI: 10.1038/srep24526 |
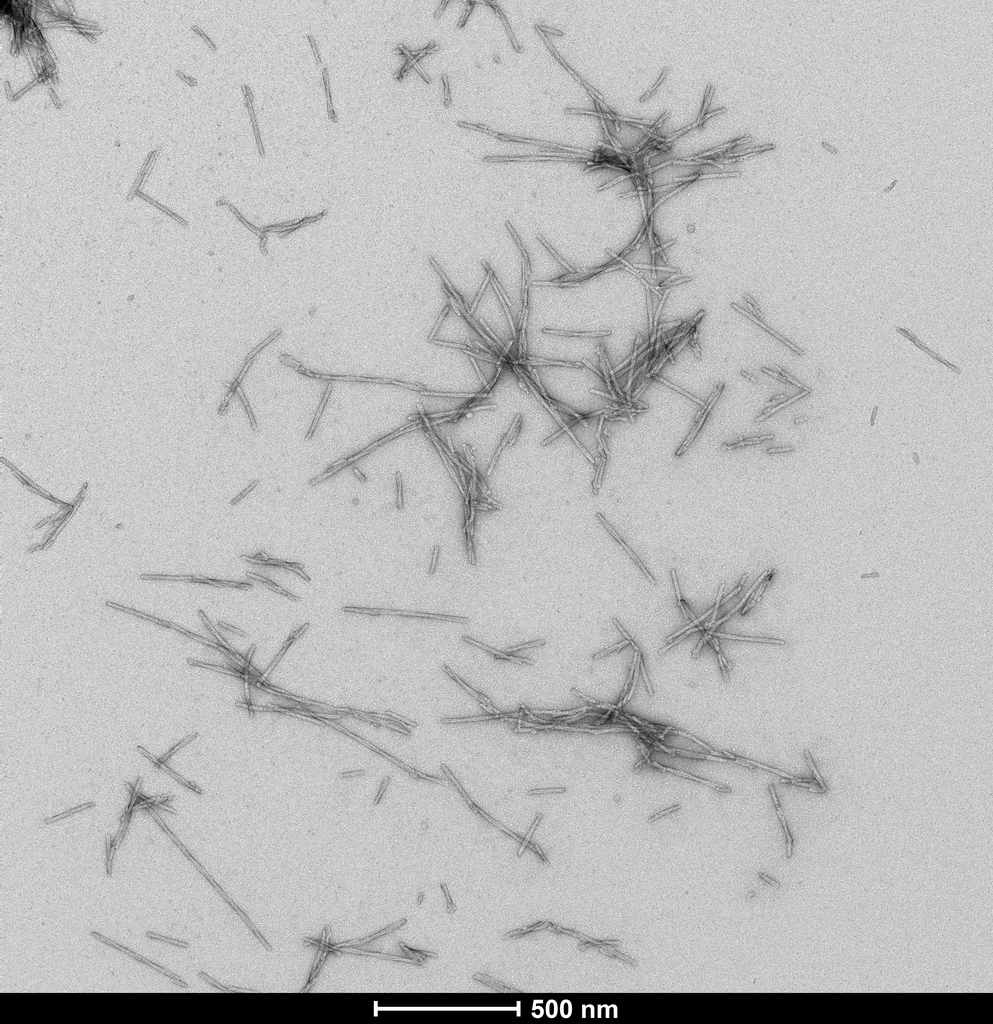
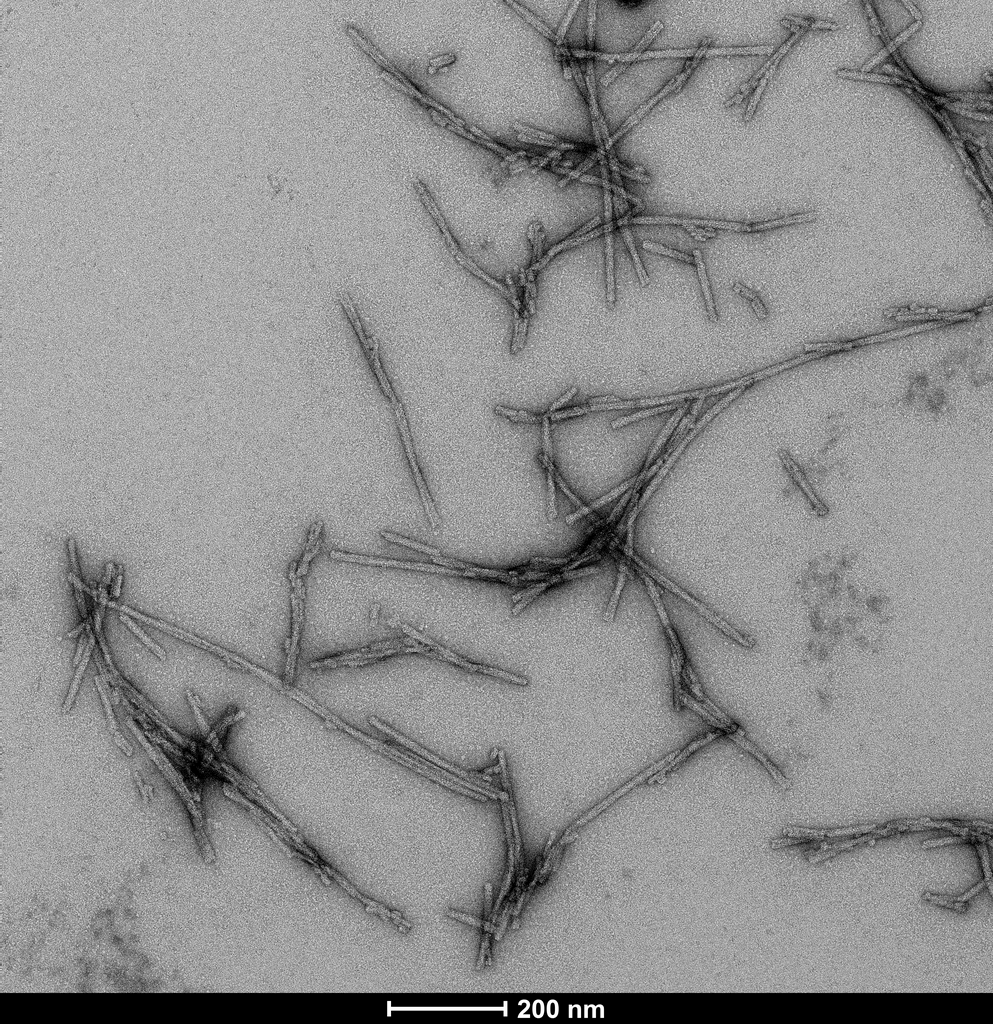
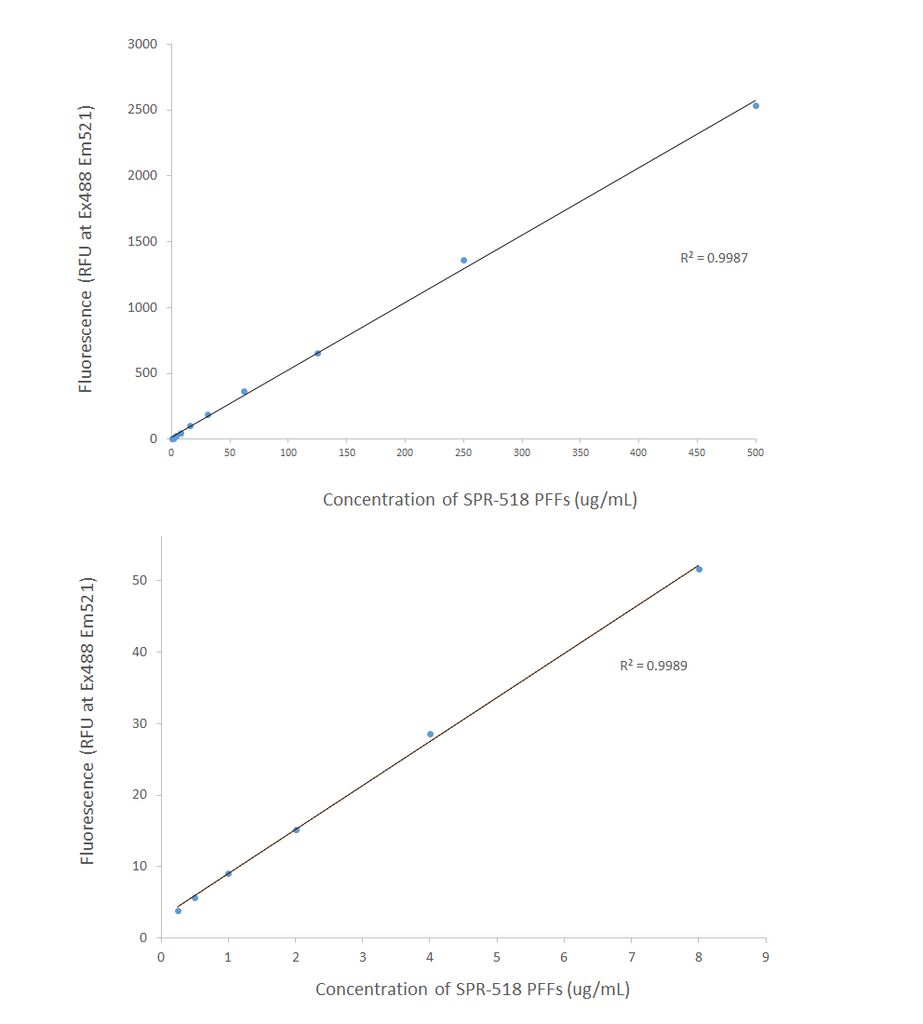
Product Images
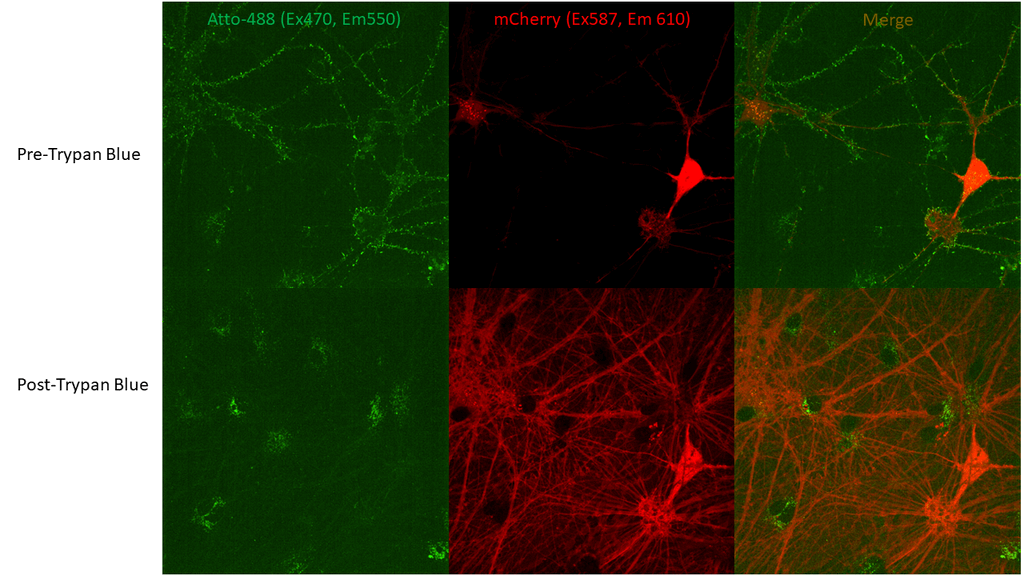
Neuronal uptake of ATTO-488 conjugated Alpha Synuclein E114C Pre-Formed Fibril (PFFs) (SPR-518-A88) visible by fluorescence after Trypan Blue quenching. Neurons expressing mCherry via AAVs (division 19) were treated with SPR-518 and then Trypan Blue to quench extracellular PFF fluorescence. ATTO-488 fluorescence present after (bottom row) Trypan Blue addition is from internalized PFFs. Note: greater mCherry signal post-Trypan Blue addition attributed to overlap of excitation/emission spectra (mCherry maxima ex 587/em610, Trypan Blue ex maxima 560/em630).
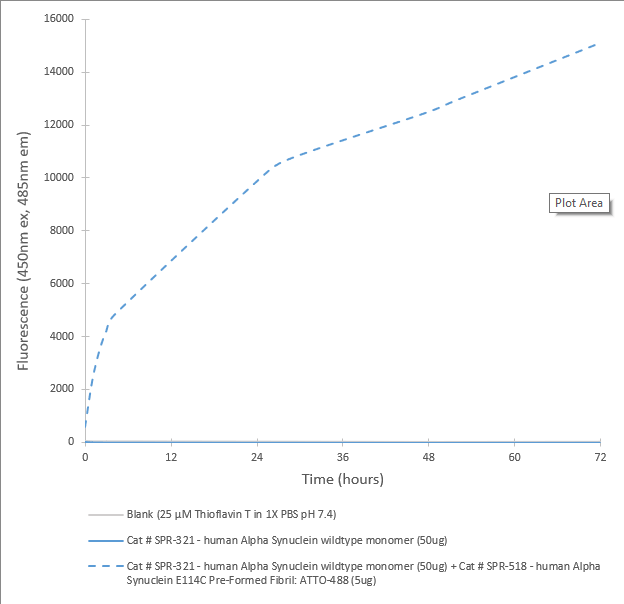
In vitro seeding activity of human Alpha Synuclein wildtype monomer in ThT assay (SPR-518-A88). SPR-518 (ATTO-488 conjugated Alpha Synuclein E114C fibrils) seed fibril formation of SPR-321 (Type 1 Alpha Synuclein wildtype monomer) over 72 hours. Reactions (100uL) shaken at 600 rpm in Greiner-Bio 96 Well Non-Binding Cell Culture Microplates, Black (Greiner-Bio Catalog #655900) at 37oC and read with an XPS Microplate Reader set at 450nmex/485nmem.
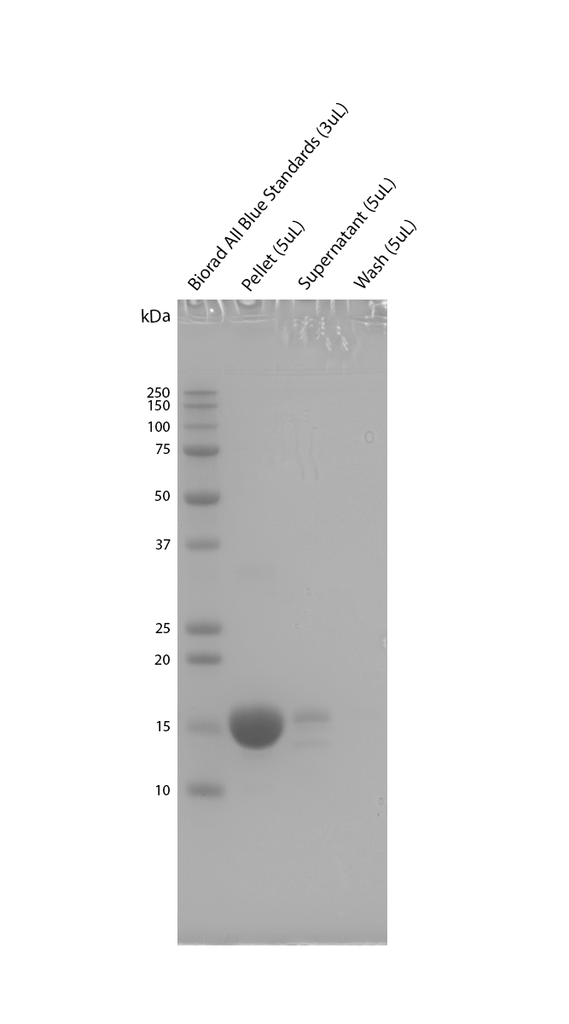
Sedimentation assay on ATTO-488 conjugated Alpha Synuclein E114C Pre-Formed Fibrils (SPR-518-A88). Samples were pelleted at 15,000 x g, washed, and then re-centrifuged. Fibril samples are prepared in denaturing conditions prior to running on the gel. SDS-PAGE analysis on a 12% Bis-Tris gel shows that the majority of fibril is insoluble.





















Reviews
There are no reviews yet.