Properties
| Storage Buffer | PBS pH 7.4 |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
| Cite This Product | Human Recombinant Tau Protein (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog #SPR-445 ) |
| Certificate of Analysis | Certified >95% pure using SDS-PAGE analysis. |
| Other Relevant Information | For corresponding PFFs, see catalog# SPR-461 |
Biological Description
| Alternative Names | Tau monomer, Tau protein monomer, Tau protein, microtubule-associated protein Tau, MAPT, MAP, microtubule-associated protein, Truncated Tau Protein Monomer, Paired Helical Filament-Tau, Phf-Tau, Neurofibrillary Tangle Protein, G Protein Beta1/Gamma2 Subunit-Interacting Factor 1, Isoform 2, tubulin-associated unit, 95-amino acid Tau protein fragment, Truncated Tau Protein |
| Research Areas | Alzheimer's Disease, Axon Markers, Cell Markers, Cell Signaling, Cytoskeleton, Microtubules, MT Associated Proteins, Neurodegeneration, Neuron Markers, Neuroscience, Tangles & Tau |
| Cellular Localization | Axolemma, Axolemma Plasma Membrane, Axon, Cell Body, Cell membrane, Cytoplasm, Cytoplasmic Ribonucleoprotein Granule, Cytoplasmic Side, Cytoskeleton, Cytosol, Dendrite, Growth cone, Microtubule, Microtubule Associated Complex, Neurofibrillary Tangle, Neuronal Cell Body, Nuclear Periphery, Nuclear Speck, Nucleus, Peripheral membrane protein, Plasma Membrane, Tubulin Complex |
| Accession Number | NP_005901.2 |
| Gene ID | 4137 |
| Swiss Prot | P10636 |
| Scientific Background | Alzheimer’s Disease (AD) is the most common neurodegenerative disease, affecting 10% of seniors over the age of 65 (1). It was named after Alois Alzheimer, a German scientist who discovered tangled bundles of fibrils where neurons had once been in the brain of a deceased patient in 1907 (2). Tau (tubulin-associated unit) is normally located in the axons of neurons where it stabilizes microtubules. Tauopathies such as AD are characterized by neurofibrillary tangles containing paired helical filaments (PHFs). A truncated 95-amino acid fragment corresponding to residues 297-391 of full-length tau has been shown to assemble into PHF-like fibrils in vitro in the absence of additives or templates (3). This fragment has been found in the core of PHFs from AD brains and forms filaments that closely resemble PHFs isolated from AD brains (3). The C322A mutation leads to enhanced self-assembly into long and ordered PHFs (3). |
| References |
1. www.alz.org/alzheimers-dementia/facts-figures 2. Alzheimer, A. Über eine eigenartige Erkrankung der Hirnrinde. Allg. Z. Psychiatr. Psych.-Gerichtl. Med. 64, 146–148 (1907) 3. Al-Hilaly, Y.K. et al. Alzheimer's Disease-like Paired Helical Filament Assembly from Truncated Tau Protein Is Independent of Disulfide Crosslinking. J. Mol. Biol. 429(23):3650-3665 (2017) |
Product Images
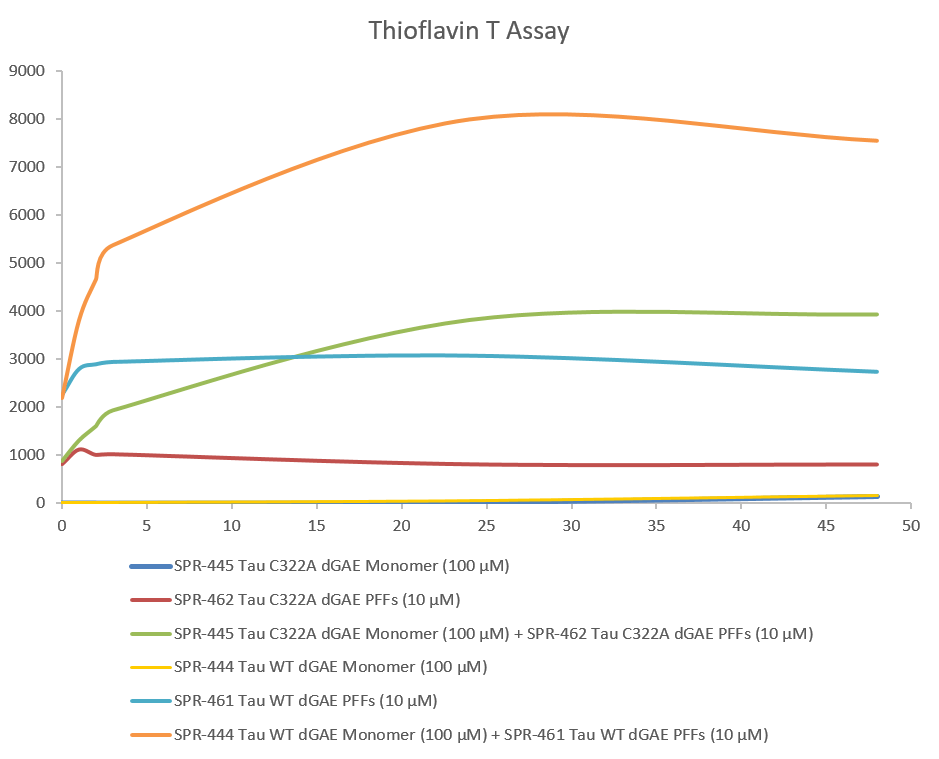
Thioflavin T is a fluorescent dye that binds to beta sheet-rich structures, such as those in tau fibrils. Upon binding, the emission spectrum of the dye experiences a red-shift and increased fluorescence intensity. Thioflavin T emission curves show increased fluorescence (correlated to tau aggregation) over time when truncated tau fragment (AA297-391) (dGAE C322A) monomer is combined with truncated tau fragment (AA297-391) (dGAE C322A) Pre-formed fibrils (Type 1).























Matthew Beckham :
Application: WB
Tissue or cell type and species: N/A
Great for recognizing Human and Mouse antigen, just what it is supposed to do.