Properties
| Storage Buffer | 10 mM Hepes pH 7.4, 100 mM NaCl |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
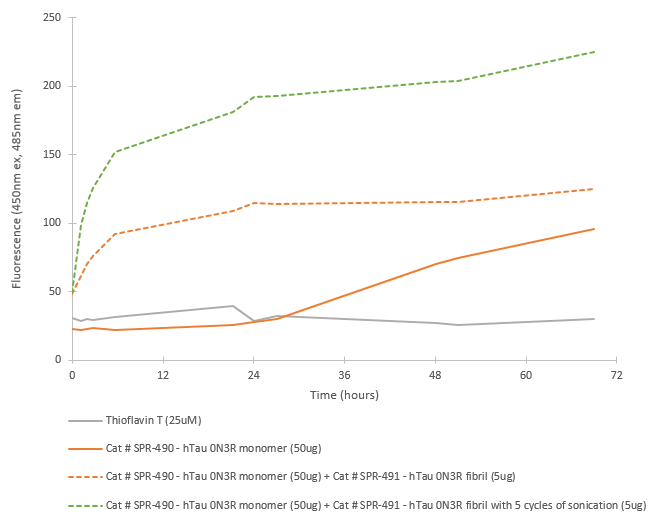
| Cite This Product | Human Recombinant Tau352 (fetal 0N3R) Monomers (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SPR-490) |
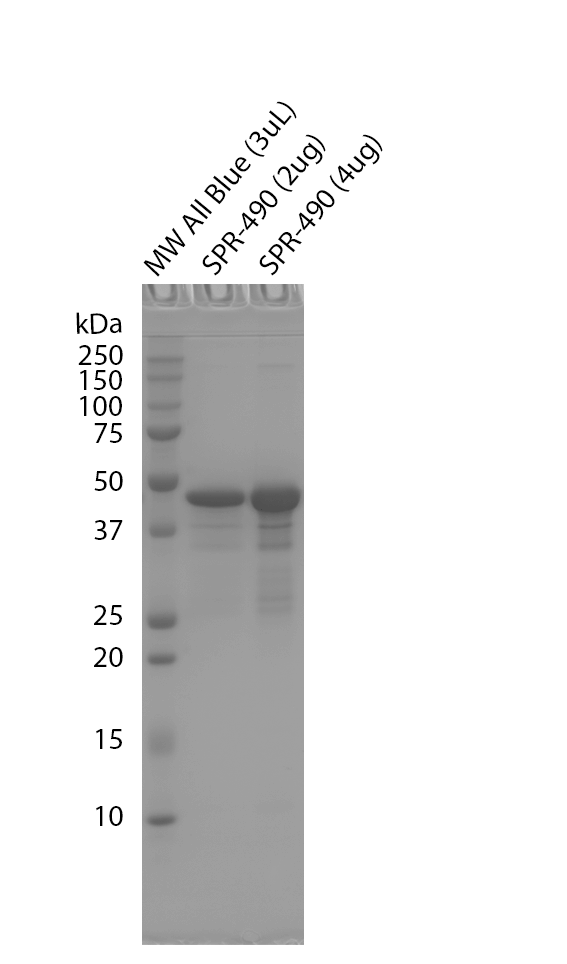
| Certificate of Analysis | Protein certified >95% pure on SDS-PAGE & Nanodrop analysis. Low endotoxin <5 EU/mL @ 2mg/mL. |
| Other Relevant Information | For corresponding PFFs, see catalog# SPR-491 |
Biological Description
| Alternative Names | Tau aggregate, tau protein, microtubule-associated protein tau, MAPT, MAP, microtubule-associated protein, Truncated Tau Protein Aggregate, Paired Helical Filament-Tau, Phf-Tau, Neurofibrillary Tangle Protein, G Protein Beta1/Gamma2 Subunit-Interacting Factor 1, Isoform 2, tubulin-associated unit, 95-amino acid tau protein fragment, Truncated Tau |
| Research Areas | Alzheimer's Disease, Axon Markers, Cell Markers, Cell Signaling, Cytoskeleton, Microtubules, MT Associated Proteins, Neurodegeneration, Neuron Markers, Neuroscience, Tangles & Tau |
| Cellular Localization | Axolemma, Axolemma Plasma Membrane, Axon, Cell Body, Cell membrane, Cytoplasm, Cytoplasmic Ribonucleoprotein Granule, Cytoplasmic Side, Cytoskeleton, Cytosol, Dendrite, Growth cone, Microtubule, Microtubule Associated Complex, Neurofibrillary Tangle, Neuronal Cell Body, Nuclear Periphery, Nuclear Speck, Nucleus, Peripheral membrane protein, Plasma Membrane, Tubulin Complex |
| Accession Number | NP_058525.1 |
| Gene ID | 4137 |
| Swiss Prot | P10636-2 |
| Scientific Background | Alzheimer’s Disease (AD) is the most common neurodegenerative disease, affecting 10% of seniors over the age of 65 (1). Tau (tubulin-associated unit) is normally located in the axons of neurons where it stabilizes microtubules. Tauopathies such as AD are characterized by neurofibrillary tangles containing paired helical filaments (PHFs). Brain-specific tau isoforms vary in the number of N-terminal inserts and C- terminal repeat domains due to alternative splicing of exons; only the shortest isoform of tau, 0N3R, is expressed in the fetal brain during neurogenesis (2). Three-repeat (3R) isoforms have been shown to be more prone than four-repeat (4R) isoforms to form oligomers in vitro (3). The β-sheet core of Tau 0N3R fibrilized using heparin differs from all other tau fibril structures known to date (4). |
| References |
1. www.alz.org/alzheimers-dementia/facts-figures. 2. Goedert et al. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrilary tangles of Alzheimer’s disease. Neuron. 1989;3(4):519-526. 3. Shahpasand-Kroner et al. Three-repeat and four-repeat tau isoforms for different oligomers. Prot. Sci. 2021;doi: 10.1002/pro4257. 4. Dregni, et al. Inclusion of the C‑Terminal Domain in the β‑Sheet Core of Heparin-Fibrillized Three-Repeat Tau Protein Revealed by Solid-State Nuclear Magnetic Resonance Spectroscopy. JACS. 2021. https://doi.org/10.1021/jacs.1c03314. |





















Reviews
There are no reviews yet.