Properties
| Storage Buffer | PBS pH 7.4 |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
| Cite This Product | Human Recombinant Alpha Synuclein Monomers (StressMarq Biosciences | Victoria, BC CANADA | Catalog# SPR-316) |
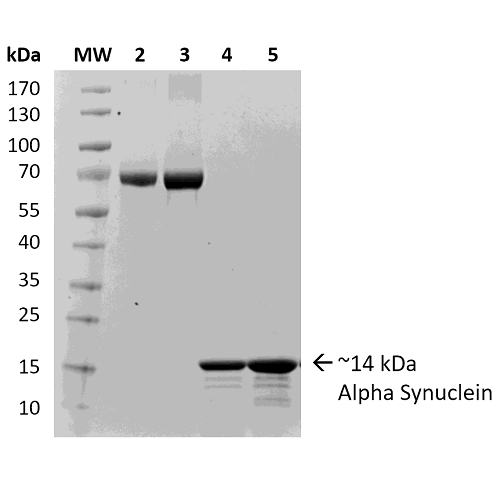
| Certificate of Analysis | Certified 95% pure using SDS-PAGE analysis. Low endotoxin <5 EU/mL @ 2mg/mL. |
| Other Relevant Information | For corresponding PFFs, see catalog# SPR-317 |
Biological Description
| Alternative Names | Alpha synuclein monomer, Alpha-synuclein monomer, Alpha synuclein protein monomer, Alpha synuclein monomer, Alpha-synuclein protein, Non-A beta component of AD amyloid protein, Non-A4 component of amyloid precursor protein, NACP protein, SNCA protein, NACP protein, PARK1 protein, Alpha synuclein monomers, SYN protein, Parkinson disease familial 1 Protein |
| Research Areas | Alzheimer's Disease, Neurodegeneration, Neuroscience, Parkinson's Disease, Synuclein, Tangles & Tau, Multiple System Atrophy |
| Cellular Localization | Cytoplasm, Membrane, Nucleus |
| Accession Number | NP_000336.1 |
| Gene ID | 6622 |
| Swiss Prot | P37840 |
| Scientific Background |
Alpha-Synuclein (SNCA) is expressed predominantly in the brain, where it is concentrated in presynaptic nerve terminals (1). Alpha-synuclein is highly expressed in the mitochondria of the olfactory bulb, hippocampus, striatum and thalamus (2). Functionally, it has been shown to significantly interact with tubulin (3), and may serve as a potential microtubule-associated protein. It has also been found to be essential for normal development of the cognitive functions; inactivation may lead to impaired spatial learning and working memory (4). SNCA fibrillar aggregates represent the major non A-beta component of Alzheimers disease amyloid plaque, and a major component of Lewy body inclusions, and Parkinson's disease. Parkinson's disease (PD) is a common neurodegenerative disorder characterized by the progressive accumulation in selected neurons of protein inclusions containing alpha-synuclein and ubiquitin (5, 6). |
| References |
1. “Genetics Home Reference: SNCA”. US National Library of Medicine. (2013). 2. Zhang L., et al. (2008) Brain Res. 1244: 40-52. 3. Alim M.A., et al. (2002) J Biol Chem. 277(3): 2112-2117. 4. Kokhan V.S., Afanasyeva M.A., Van'kin G. (2012) Behav. Brain. Res. 231(1): 226-230. 5. Spillantini M.G., et al. (1997) Nature. 388(6645): 839-840. 6. Mezey E., et al. (1998) Nat Med. 4(7): 755-757. |
Product Images
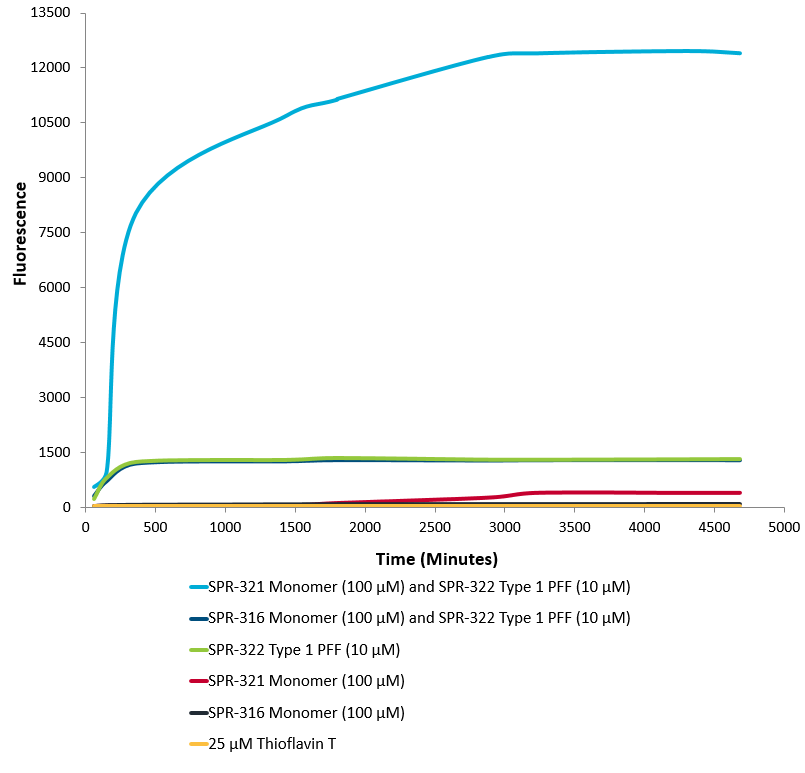
Thioflavin T is a fluorescent dye that binds to beta sheet-rich structures, such as those in alpha synuclein fibrils. Upon binding, the emission spectrum of the dye experiences a red-shift and increased fluorescence intensity. Thioflavin T emission curves show increased fluorescence (correlated to alpha synuclein protein aggregation) over time when 10 µM of Type 1 alpha synuclein Pre-formed fibrils (SPR-322) is combined with 100 µM of Type 1 alpha synuclein monomer (SPR-321), compared to 10 µM of Type 1 alpha synuclein Pre-formed fibrils (SPR-322) combined with 100 µM Type 2 alpha synuclein monomer (SPR-316). Type 2 fibrils (SPR-317) do not seed type 2 monomers (SPR-316) (data not shown). Thioflavin T ex = 450 nm, em = 485 nm. Note: We use molecular weight of 14.46 kDa for both alpha synuclein monomer and fibril in calculations. We load 100µL/well for Thioflavin T assay so 100 µM is 144.6µg/well and 10 µM is 14.46 µg/well.
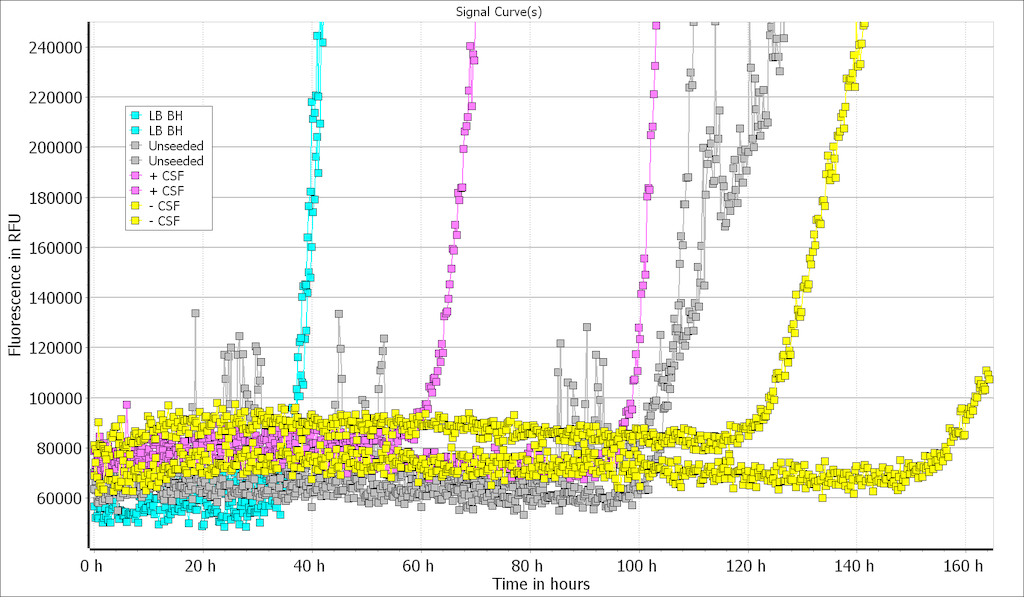
Type 2 monomers (SPR-316) are currently undergoing testing in a Real-Time Quaking-Induced Conversion (RT-QuIC) assay. At 10 μg/well there was discrimination between positive and negative CSF samples, and the unseeded reaction occurred later than either the LB BH or the positive CSF sample. This suggests Type 2 monomers could potentially be used as a substrate for alpha synuclein RT-QuIC. Further testing/optimization is underway. LB BH: 10% Lewy body disease; CSF +: CSF from patient with neuropathologically confirmed alpha-synucleinopathy; CSF -: CSF from patient with no evidence of alpha-synuclein deposition at postmortem. Image source: Alison Green, Graham Fairfoul
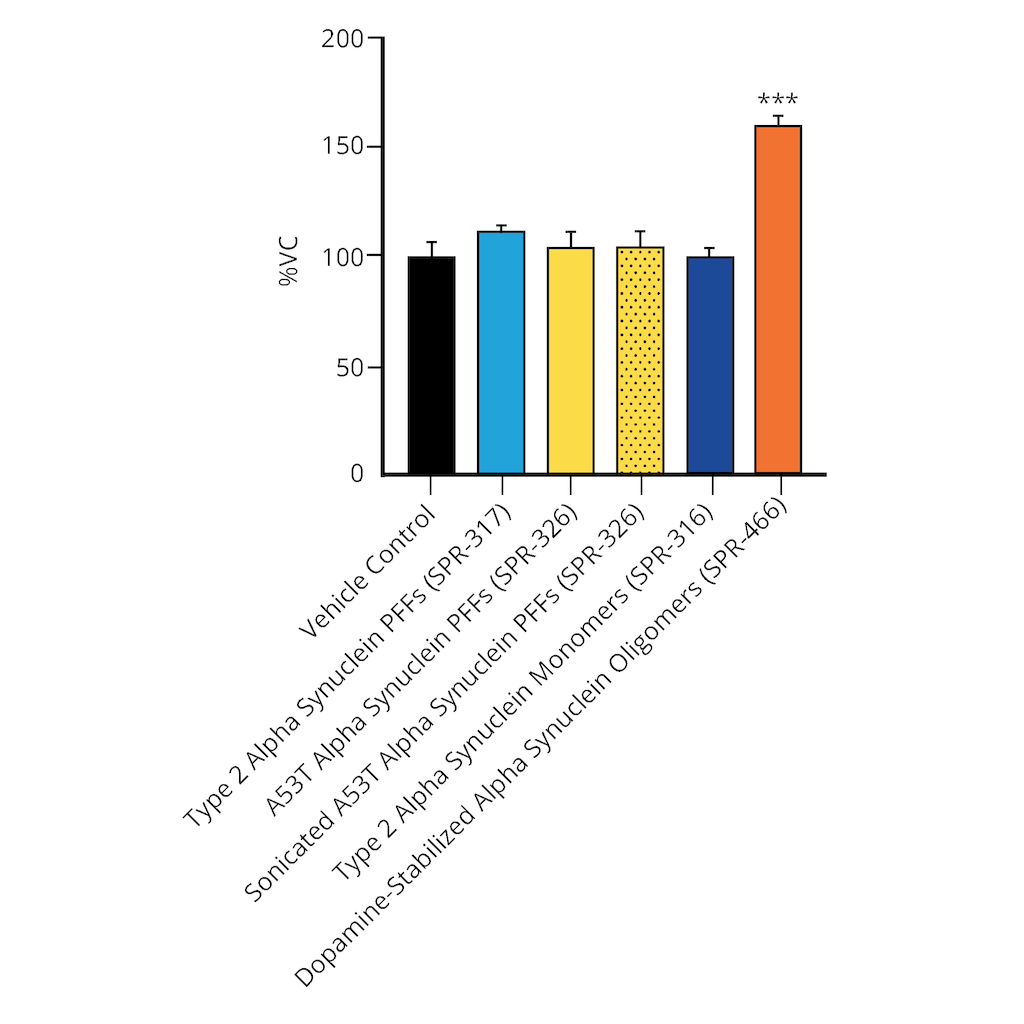
Evaluation of a-syn toxicity on primary mouse cortical neurons. Lactate dehydrogenase (LDH) is a soluble enzyme present in the cytosol that is released upon cell death. Toxicity was assessed with an LDH assay and displayed as % of vehicle control (VC). Data are presented as bar graphs and standard deviation. For statistical analysis One-way ANOVA followed by Bonferroni post-hoc test (vs VC) was used. *** p<0.001. Treatment with alpha synuclein monomers did not significantly impact LDH release. Data courtesy of QPS.
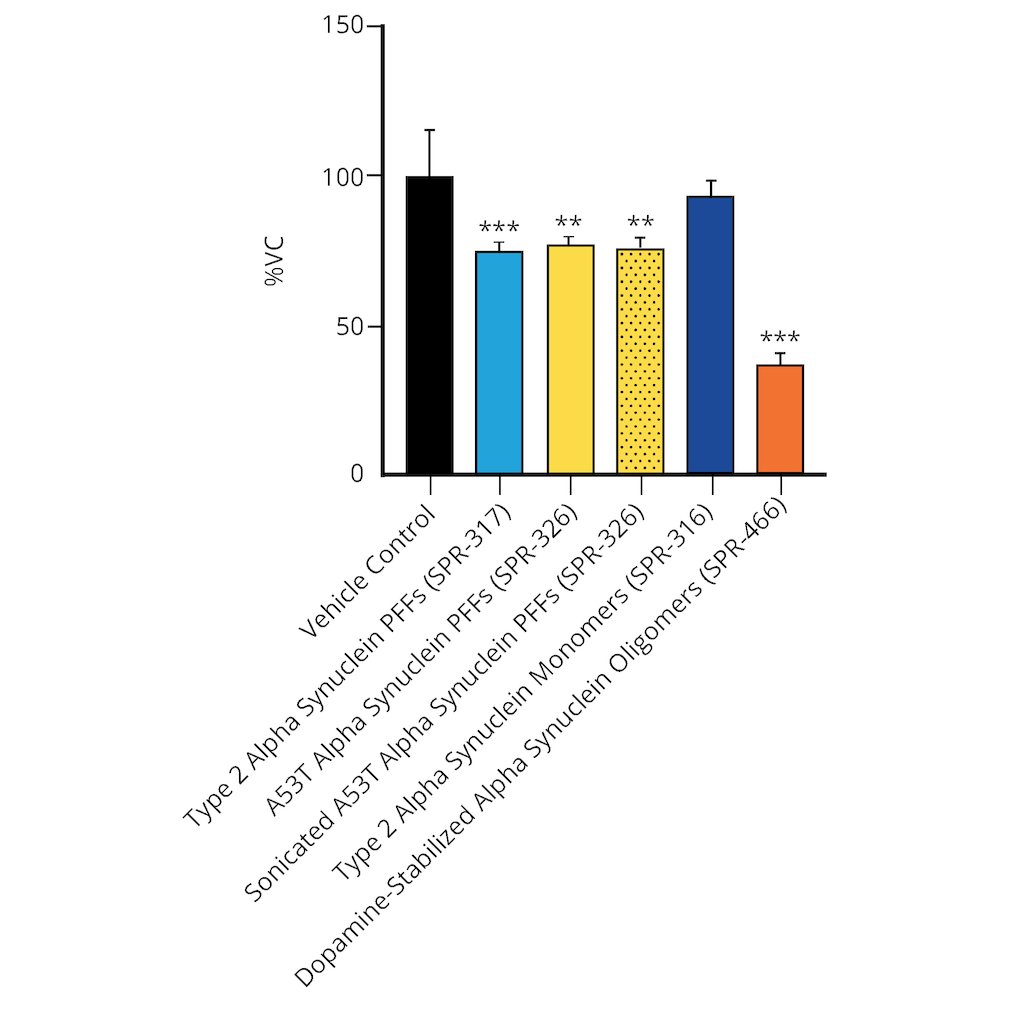
Evaluation of a-syn toxicity on primary mouse cortical neurons. Mitochondrial dehydrogenase activity reduces yellow MTT to dark blue formazan crystals, a reaction catalyzed in living cells. Cell viability was assessed with an MTT assay and displayed as % of vehicle control (VC). Data are presented as bar graphs and standard deviation. For statistical analysis One-way ANOVA followed by Bonferroni post-hoc test (vs VC) was used. ** p<0.01, *** p<0.001. Treatment with alpha synuclein monomers did not significantly impact cell viability. Data courtesy of QPS.
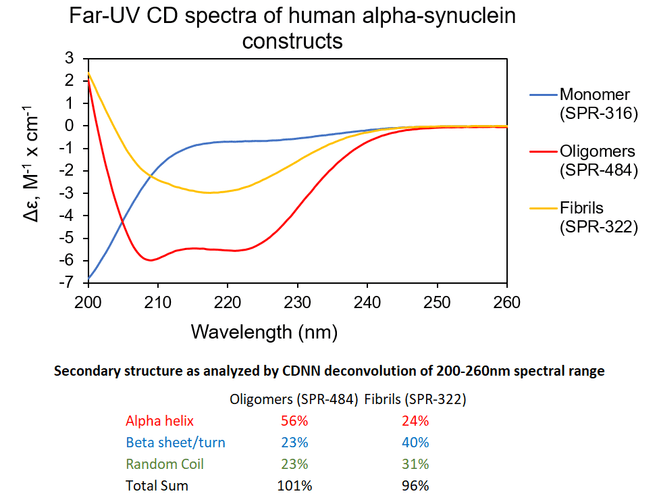
Αlpha Synuclein Oligomers Have Distinct Secondary Structure Differences Compared to Fibrils. UV-CD data suggests that StressMarq’s Alpha Synuclein Oligomers have distinct secondary structure differences compared to our monomers and fibrils. More specifically, StressMarq’s Kinetically Stable Alpha Synuclein Oligomers (SPR-484) show a significantly higher alpha helix content and lower beta sheet/turn content than our Alpha Synuclein Pre-formed Fibrils (Type 1) (SPR-322). StressMarq’s Alpha Synuclein Monomers (SPR-316) show a strong negative signal at 200 nm indicative of a disordered protein state (low secondary structure content).
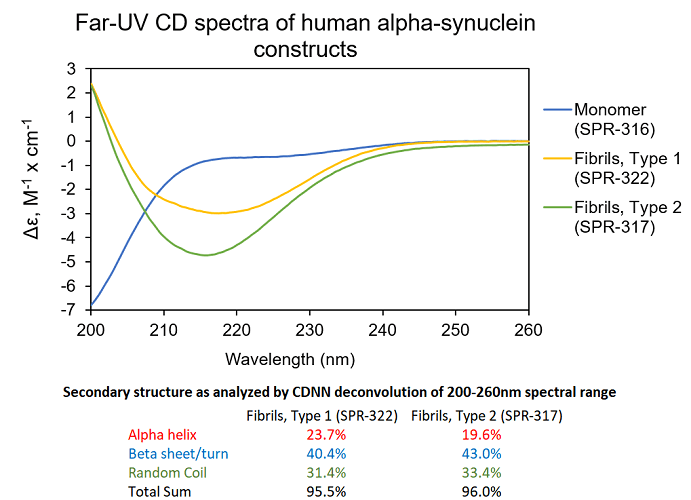
UV-CD data suggests StressMarq’s Alpha Synuclein Pre-formed Fibrils (PFF), Type 1 (cat#SPR-322) and Type 2 (cat#SPR-317) both have a high beta sheet/turn content, yet do have small secondary structure differences. StressMarq’s Alpha Synuclein Monomers (cat#SPR-316) show a strong negative signal at 200 nm indicative of a disordered protein state (low secondary structure content). For this experiment, pre-formed fibrils (PFF) were subjected to 10 cycles of sonication prior to UV-CD to ensure solubility prior to measurement.

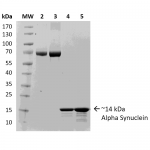

























Reviews
There are no reviews yet.