Properties
| Storage Buffer | PBS pH 7.4 |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | Ion-exchange Purified |
| Cite This Product | Mouse Recombinant Alpha Synuclein Protein (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SPR-323) |
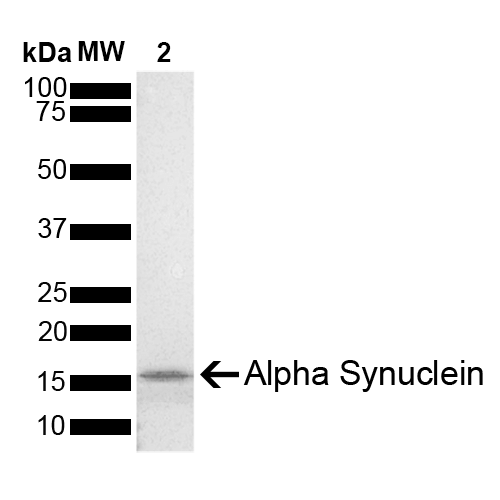
| Certificate of Analysis | Certified >95% pure using SDS-PAGE analysis. Low endotoxin <5 EU/mL @ 2mg/mL. |
| Other Relevant Information | For corresponding PFFs, see catalog# SPR-324 |
Biological Description
| Alternative Names | Alpha synuclein monomer, Alpha-synuclein monomer, Alpha synuclein protein monomer, Alpha synuclein monomer, Alpha-synuclein protein, Non-A beta component of AD amyloid protein, Non-A4 component of amyloid precursor protein, NACP protein, SNCA protein, NACP protein, PARK1 protein, Alpha synuclein monomers, SYN protein, Parkinson's disease familial 1 Protein |
| Research Areas | Alzheimer's Disease, Neurodegeneration, Neuroscience, Parkinson's Disease, Synuclein, Tangles & Tau, Multiple System Atrophy |
| Cellular Localization | Cytoplasm, Membrane, Nucleus |
| Accession Number | NP_001035916.1 |
| Gene ID | 20617 |
| Swiss Prot | O55042 |
| Scientific Background | Alpha-Synuclein (SNCA) is expressed predominantly in the brain, where it is concentrated in presynaptic nerve terminals (1). Alpha-synuclein is highly expressed in the mitochondria of the olfactory bulb, hippocampus, striatum and thalamus (2). Functionally, it has been shown to significantly interact with tubulin (3), and may serve as a potential microtubule-associated protein. It has also been found to be essential for normal development of the cognitive functions; inactivation may lead to impaired spatial learning and working memory (4). SNCA fibrillar aggregates represent the major non A-beta component of Alzheimers disease amyloid plaque, and a major component of Lewy body inclusions, and Parkinson's disease. Parkinson's disease (PD) is a common neurodegenerative disorder characterized by the progressive accumulation in selected neurons of protein inclusions containing alpha-synuclein and ubiquitin (5, 6). |
| References |
1. “Genetics Home Reference: SNCA”. US National Library of Medicine. (2013). 2. Zhang L., et al. (2008) Brain Res. 1244: 40-52. 3. Alim M.A., et al. (2002) J Biol Chem. 277(3): 2112-2117. 4. Kokhan V.S., Afanasyeva M.A., Van'kin G. (2012) Behav. Brain. Res. 231(1): 226-230. 5. Spillantini M.G., et al. (1997) Nature. 388(6645): 839-840. 6. Mezey E., et al. (1998) Nat Med. 4(7): 755-757. |
Product Images
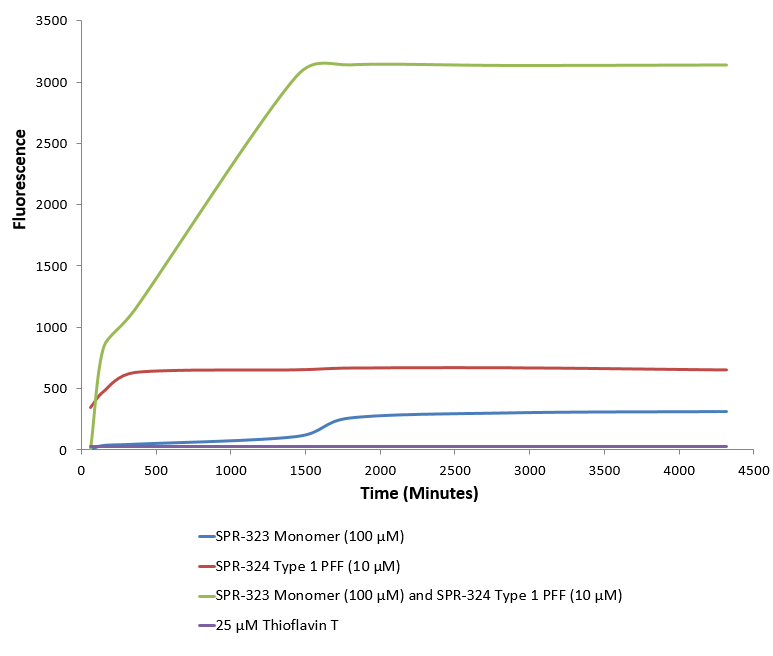
Type 1 alpha synuclein pre-formed fibrils (SPR-324) seed the formation of new alpha synuclein fibrils from the pool of alpha synuclein monomers (SPR-323). Thioflavin T is a fluorescent dye that binds to beta sheet-rich structures, such as those in alpha synuclein fibrils. Upon binding, the emission spectrum of the dye experiences a red-shift, and increased fluorescence intensity. Thioflavin T emission curves show increased fluorescence (correlated to alpha synuclein protein aggregation) over time when 10 µM of Type 1 alpha synuclein pre-formed fibrils (SPR-324) is combined with 100 µM of alpha synuclein monomer (SPR-323), as compared to Type 1 alpha synuclein pre-formed fibrils (SPR-324) or alpha synuclein monomer (SPR-323) alone. Thioflavin T ex = 450 nm, em = 485 nm.
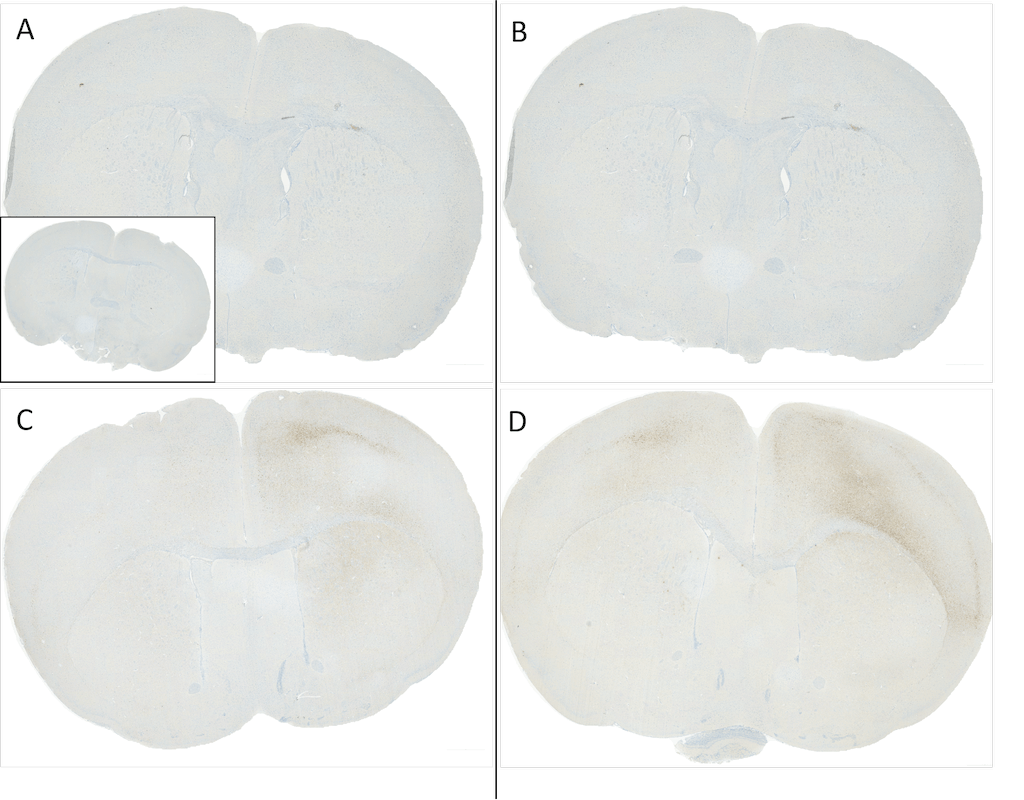
C57/BL6 mice were injected with sonicated recombinant mouse alpha synuclein monomers or fibrils at 8 weeks of age. Mice were unilaterally injected in the dorsal striatum (bregma AP + 0.2 mm, L +/1 2.0 mm, V – 3.0 mm) and sacrificed 30 days post-injection. (A) 1.25 uL mouse alpha synuclein monomers (SPR-323). (B) 2.5 uL mouse alpha synuclein monomers (SPR-323). (C) 2.5 ug alpha synuclein PFFs (SPR-324). (C) 5 ug alpha synuclein PFFs (SPR-324) Inset: PBS (negative control). Primary antibody: Anti-Alpha Synuclein pSer129 (SMC-600) at 1:10 000. Secondary antibody: anti-rabbit HRP. Mice injected with PFF displayed alpha synuclein staining in the striatum and cortex and contralateral to the injection site. Courtesy of: Porsolt.























Reviews
There are no reviews yet.