Properties
| Storage Buffer | Phosphate buffer (PB) pH 7.4 and 10 mM NaCl |
| Storage Temperature | -80ºC |
| Shipping Temperature | Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order. |
| Purification | N/A |
| Cite This Product | Human Synthetic Amyloid Beta Oligomers (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SPR-488) |
| Certificate of Analysis | Certified >95% pure using mass spec and HPLC. Low endotoxin <2.5 EU/mL @ 1mg/mL. |
Biological Description
| Alternative Names | Abeta Oligomers, Abeta peptide, Amyloid beta peptide oligomers, Beta amyloid peptide oligomers, amyloid beta precursor protein peptide oligomers, APP |
| Research Areas | Alzheimer's Disease, Amyloid, Neurodegeneration, Neuroscience |
| Cellular Localization | Cell membrane, Intracellular Vesicles |
| Gene ID | 351 |
| Swiss Prot | P05067 |
| Scientific Background | Our Amyloid Beta 1-42 (Aβ42) Oligomers are generated from Amyloid Beta Peptide 1-42 pre-treated with 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) as previously published (1,2). Our Aβ42 oligomers present as globular oligomers when observed under TEM and AFM, and have a unique dimer/trimer and oligomer signal on a Western Blot with an anti-amyloid beta antibody. Our Aβ42 oligomers were also demonstrated to be toxic to primary rat cortical neurons in a dose-dependent manner. In the brain, amyloid beta peptide (Aβ) is generated by protease cleavage of amyloid precursor protein (APP), which aggregates into oligomers, protofibrils, fibrils and ultimately plaques in neurodegenerative diseases. The accumulation of Aβ plaques in the brain is considered a hallmark of Alzheimer’s disease (AD), and most of the drugs tested for AD in the past 20 years have targeted amyloid beta accumulation (3). Soluble Aβ oligomers isolated from the brains of AD patients or those generated in vitro potently impaired synapse structure and function (4). Aβ oligomers generated in vitro were toxic to PC12 cells (5) and SH-SY5Y cells (6). Aβ was demonstrated to interact with tauopathies to affect neurodegeneration in AD patients (7) and accumulations of Aβ were shown to be associated with lower survival rates in Parkinson’s disease patients with dementia (8). |
| References |
1. Stine et al. 2003. JBC. 278(13):11612-22. doi: 10.1074/jbc.M210207200 2. Ahmed et al. 2010. Nature Structural & Molecular Biology. 17(5):561-7. doi: 10.1038/nsmb.1799 3. Panza et al. 2019. Nat Rev Neurol. 15:73-88 https://doi.org/10.1038/s41582-018-0116-6 4. Shankar et al. 2008. Nat Med. 14(8):837-842. doi: 10.1038/nm1782 5. Chromy et al. 2003. Biochemistry. 42:12749-12760. doi: 10.1021/bi030029q 6. Kayed et al. 2003. Science. 300(5618): 486-489. doi: 10.1126/science.1079469 7. Want et al. 2016. JAMA Neurol. 73(9):1070-7. doi: 10.1001/jamaneurol.2016.2078 8. Kotzbauer et al. 2012. Arch Neurol. 69(10): 1326-1331. doi: 10.1001/archneurol.2012.1608 |
Product Images

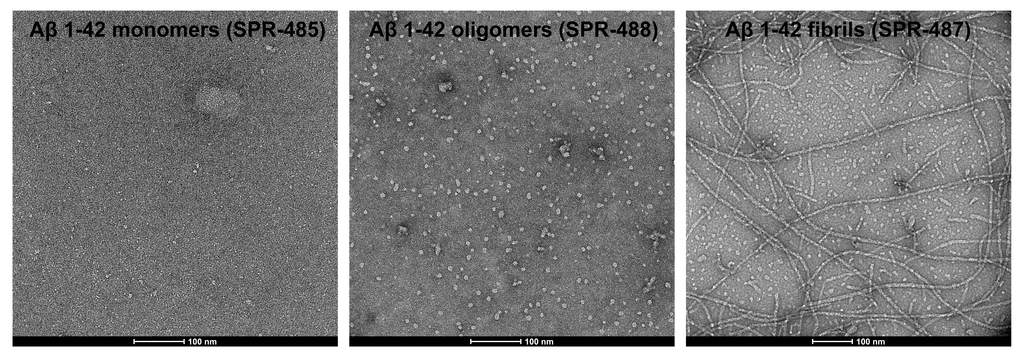
AFM of amyloid beta 1-42 monomers (SPR-485, left), oligomers (SPR-488, middle) and fibrils (SPR-487, right). Atomic force microscopy analysis of 1.0 mg/mL samples diluted to 0.1 mg/mL in dH2O, mounted on freshly cleaved mica, washed, dried and analyzed with tapping mode. Representative images are 2.5 x 2.5 µm x-y with a z-range of 10 nm.
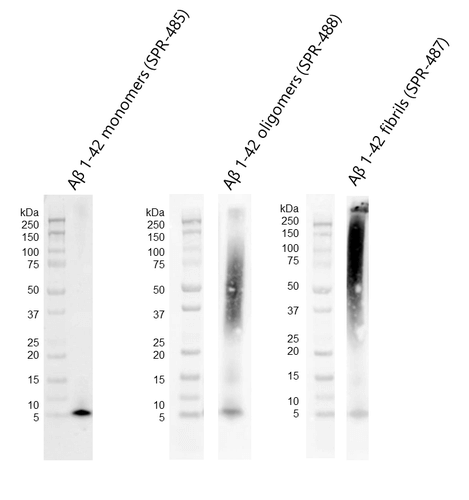
Western blot of amyloid beta 1-42 monomers (SPR-485, left), oligomers (SPR-488, middle) and fibrils (SPR-487, right) using anti-amyloid beta 6E10 antibody. Amyloid beta constructs at 160 pmol were run on 4-12% Bis-Tris SDS-PAGE, transferred to nitrocellulose in the presence of 0.02% v/v Tween-20, and blotted with 1:1000 mouse 6E10 primary antibody (Biolegend). Oligomers observed under TEM/AFM appear as distinct dimer/trimer bands at ~37-75 kDa on Western Blot with 6E10 antibody (middle). Fibrils observed under TEM/AFM appear as a distinct signal at greater than 100 kDa in the stacking gel (right).
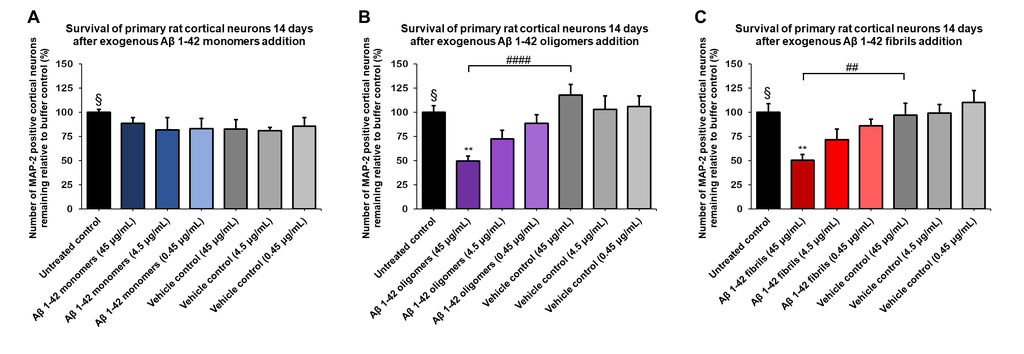
Amyloid beta 1-42 oligomers (SPR-488) and fibrils (SPR-487) show a dose-dependent toxicity to primary rat cortical neurons, but not monomers (SPR-485). Survival of rat primary cortical neurons 14 days after treatment with different concentrations of (A) monomers, (B) oligomers or (C) fibrils quantified by MAP2 positive neurons and expressed as a percentage of control. Fibrils and respective vehicle controls were initially sonicated in a Bioruptor. Test conditions were run in the same plate as untreated control and vehicle controls, which consisted of buffer without amyloid beta 1-42 protein. Data expressed as mean +/- s.e.m. (n=6). A global analysis of the data was performed using a one-way ANOVA followed by Dunnett’s test; ** p<0.01 stats vs control; ## p<0.01, #### p<0.0001 stats vs vehicle control. § represents untreated control condition.




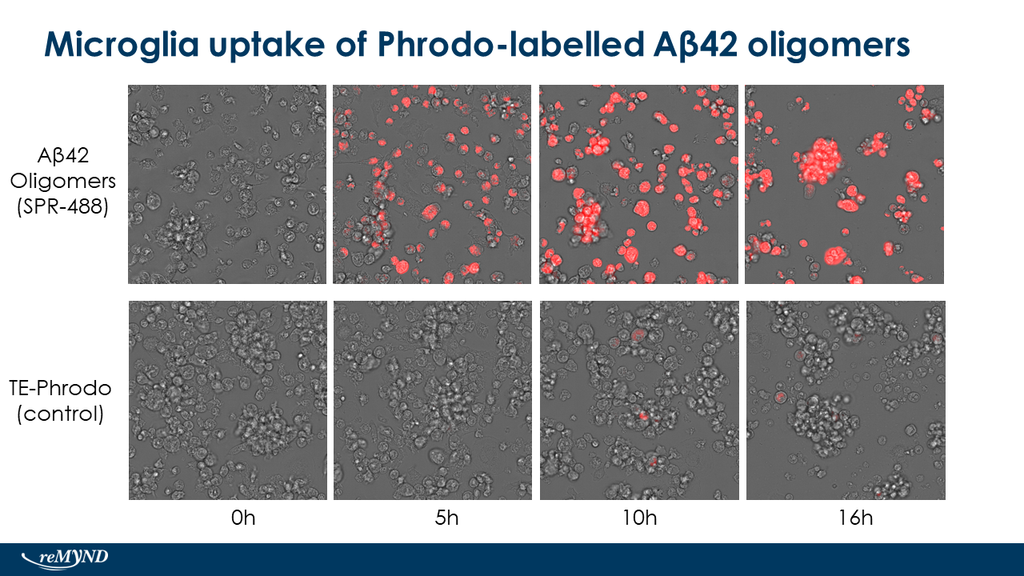





















Reviews
There are no reviews yet.