| Product Name | Flumazenil |
| Description |
Benzodiazepine antagonist |
| Purity | >98% (HPLC); NMR (conforms) |
| CAS No. | 78755-81-4 |
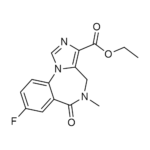
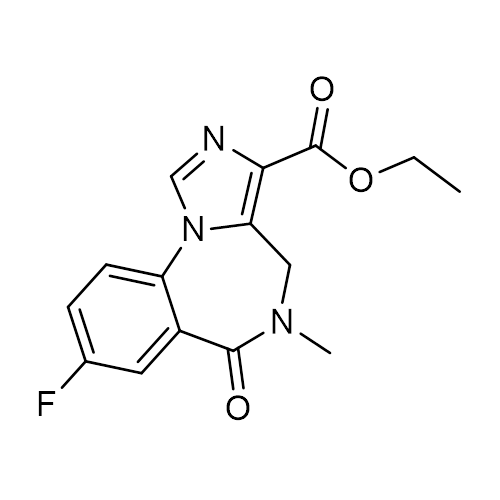
| Molecular Formula | C15H14FN3O3 |
| Molecular Weight | 303.3 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Antagonist |
| Solubility | May be dissolved in DMSO (8 mg/ml) |
| Source | Synthetic |
| Appearance | White powder |
| SMILES | CCOC(=O)C1=C2CN(C(=O)C3=C(N2C=N1)C=CC(=C3)F)C |
| InChI | InChI=1S/C15H14FN3O3/c1-3-22-15(21)13-12-7-18(2)14(20)10-6-9(16)4-5-11(10)19(12)8-17-13/h4-6,8H,3,7H2,1-2H3 |
| InChIKey | OFBIFZUFASYYRE-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Caution- Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S24/25- Avoid contact with skin and eyes |
| Cite This Product | Flumazenil (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-622) |
Biological Description
| Alternative Names | 8-Fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid, ethyl ester; Ro 1722; Ro 15-1788 |
| Research Areas | GABA Receptors, Neuroscience, Neurotransmission, Neurotransmitter Receptors |
| PubChem ID | 3373 |
| Scientific Background | Flumazenil is a benzodiazepine antagonist that binds to GABA_A receptors containing α1, α2, α3, or α5 subunits. It is clinically used to reverse benzodiazepine-induced sedation and toxicity. In neurodegenerative disease research, Flumazenil is studied for its effects on GABAergic signaling, withdrawal syndromes, and its potential to modulate cognitive and behavioral outcomes in CNS disorders. |
| References |
1. Möhler H., et al. (1981) J. Neurochem. 37:714. 2. Doble A., (1999) J. Psychopharmacol. 13(4 Suppl 1):S11. 3. Hood SD., et al. (2014) Br. J. Clin. Pharmacol. 77:285. 4. Lheureux P., et al.(1990) Hum. Exp. Toxicol. 9:105. 5. Masciullo M., et al. (2021) J. Med. Case Rep. 15:242. |

Reviews
There are no reviews yet.