| Product Name | Linopirdine dihydrochloride |
| Description |
KCNQ Channel Blocker |
| Purity | >98% (HPLC); NMR (conforms) |
| CAS No. | 113168-57-3 |
| Molecular Formula | C26H23Cl2N3O |
| Molecular Weight | 464.4 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Blocker |
| Solubility | May be dissolved in DMSO (25 mg/ml): or water (25 mg/ml) |
| Source | Synthetic |
| Appearance | Off-white powder |
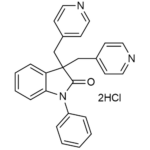
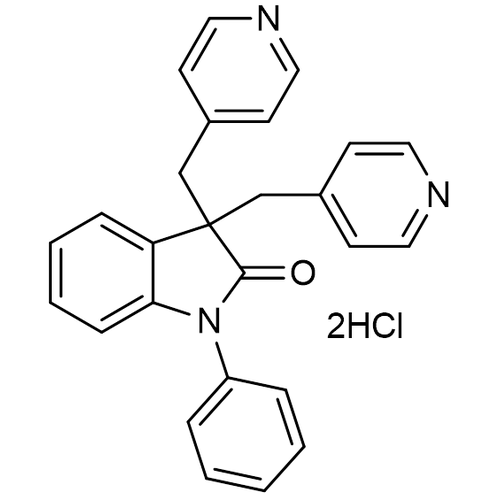
| SMILES | C1=CC=C(C=C1)N2C3=CC=CC=C3C(C2=O)(CC4=CC=NC=C4)CC5=CC=NC=C5.Cl.Cl |
| InChI | InChI=1S/C26H21N3O.2ClH/c30-25-26(18-20-10-14-27-15-11-20,19-21-12-16-28-17-13-21)23-8-4-5-9-24(23)29(25)22-6-2-1-3-7-22;;/h1-17H,18-19H2;2*1H |
| InChIKey | ZEVVHCGTTNRYOY-UHFFFAOYSA-N |
| Safety Phrases | Classification: Warning |
| Cite This Product | Linopirdine dihydrochloride (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-629) |
Biological Description
| Alternative Names | 1-Phenyl-3,3-bis(pyridine-4-ylmethyl)indolin-2-one, dihydrochloride; DuP996 |
| Research Areas | Ion Channels, Neuroscience, Neurotransmission, Potassium Channels, Voltage-Gated Potassium Channels |
| PubChem ID | 14209557 |
| Scientific Background | Linopirdine increases acetylcholine release and improves performance in animal models of learning and memory via blockade of Kv7 (KCNQ) voltage-gated potassium channels (1-3). Linopirdine is a state-dependent blocker favoring activated single subunits of the channel (4). |
| References |
1. D Kristufeck et al. J. Neurochem. 1999 72:2083 2. HS Wang et al. Science 1998 282:1890 3. DJ Fontana et al. Pharmacol. Biochem. Behav. 1994 49:1075 4. DL Greene et al. J. Pharmacol. Exp. Ther. 2017 362:177 |

Reviews
There are no reviews yet.