| Product Name | Muscone |
| Description |
Antiinflammatory and Neuroprotective activity |
| Purity | >98% (TLC); NMR (Conforms) |
| CAS No. | 541-91-3 |
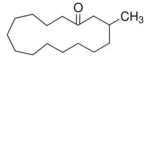
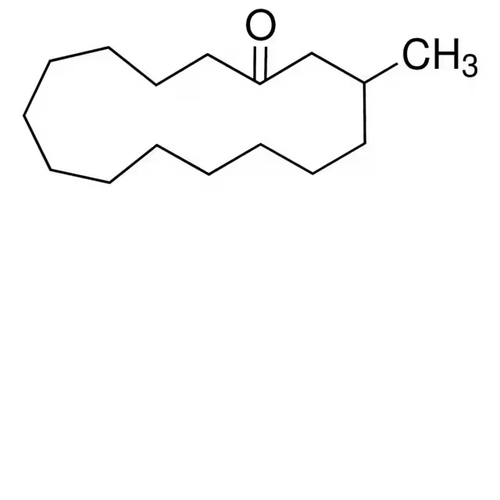
| Molecular Formula | C16H30O |
| Molecular Weight | 238.4 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Blocker |
| Solubility | May be dissolved in DMSO (35 mg/ml); or Ethanol (35 mg/ml) |
| Source | Synthetic |
| Appearance | Colorless oil |
| SMILES | CC1CCCCCCCCCCCCC(=O)C1 |
| InChI | InChI=1S/C16H30O/c1-15-12-10-8-6-4-2-3-5-7-9-11-13-16(17)14-15/h15H,2-14H2,1H3 |
| InChIKey | ALHUZKCOMYUFRB-UHFFFAOYSA-N |
| Safety Phrases | GHS Hazard Statements: Not Classified |
| Cite This Product | Muscone (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-612) |
Biological Description
| Alternative Names | 3-Methyl-cyclopentadecanone (racemic) |
| Research Areas | Angiogenesis, Cardiovascular System |
| PubChem ID | 10947 |
| Scientific Background | Muscone is a macrocyclic ketone found naturally in a glandular secretion of the musk deer which acts at human odorant receptor OR5AN1 and is used in perfumes(1). It suppresses inflammatory responses and neuronal damage in various models including a rat model of cervical spondylotic myelopathy (2) and relieves inflammatory pain acting via the NOX4/JAK2-STAT3 and NLRP3 inflammasome pathway (3). In the cardiovascular system it alleviates myocardial ischemia-reperfusion injury (4) and improves cardiac function in mice after myocardial infarction (5) via enhancement of angiogenesis (6). |
| References |
1. Ahmed L., et al. (2018) Proc. Acad. Natl. Sci. USA. 115:E3950 2. Zhou L.Y., et al. (2020) J. Neurochem. 155:154 3. Yu S., et al. (2020) Int. Immunopharmacol. 82:106355 4. Wei C.J., et al. (2021) J. Biol. Regul. Homeost. Agents. 35:85 5. Du Y., et al. (2018) Am. J. Transl. Res. 10:4235 6. Du Y., et al. (2018) Med. Sci. Monit. 24:8870 |

Reviews
There are no reviews yet.