| Product Name | Camptothecin |
| Description |
Topoisomerase I inhibitor |
| Purity | >98% |
| CAS No. | 7689-03-4 |
| Molecular Formula | C20H16N2O4 |
| Molecular Weight | 348.36 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 5 mM in DMSO |
| Source | Synthetic |
| Appearance | Yellow solid |
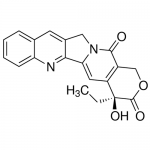
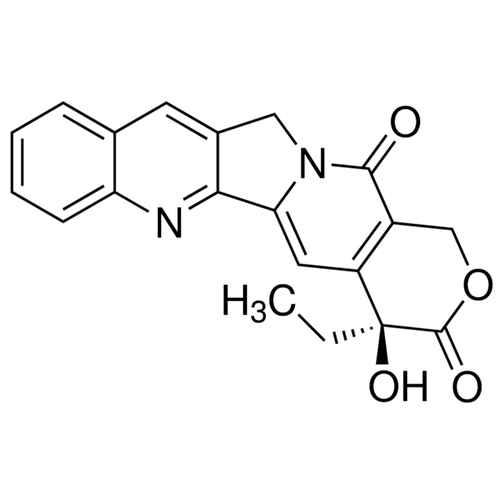
| SMILES | CC[C@@]1(C2=C(COC1=O)C(=O)N3CC4=CC5=CC=CC=C5N=C4C3=C2)O |
| InChI | InChI=1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1 |
| InChIKey | VSJKWCGYPAHWDS-FQEVSTJZSA-N |
| Safety Phrases |
Classification: Toxic. May be harmful or fatal if inhaled, swallowed or absorbed through skin. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible) Risk Phrases: R23/24/25 - Toxic by inhalation, in contact with skin and if swallowed R46 - May cause heritable genetic damage Hazard Phrases: H302 |
| Cite This Product | Camptothecin (StressMarq Biosciences, Canada, Cat # SIH-242) |
Biological Description
| Alternative Names | (4S)-4-Ethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione |
| Research Areas | Apoptosis, Cancer |
| PubChem ID | 24360 |
| Scientific Background | Camptothecin is a cytotoxic quinoline alkaloid which inhibits the DNA enzyme topoisomerase I. It binds to the topo I and DNA complex, resulting in a tertiary comlex which stabilizes it. This prevents re-ligation and therefore causes DNA damage which results in apoptosis (1-3). It has showed remarkable anti-cancer activity in clinical trials. |
| References |
1. Adams D.J., et al. (2005) Cancer Chem and Pharm. 57(2): 145-154. 2. Redinbo M.R., Stewart L., Kuhn P., Champoux J.J. and Hol W.G.J. (1998) Science. 279:1504-1513. 3. Pommier Y., et al. (2003) Mutat Res. 532: 173-203. |

Reviews
There are no reviews yet.