| Product Name | FCR1 |
| Description |
FRET ratiometric fluorescence-based redox sensor |
| Molecular Formula | C34H37N7O6 |
| Molecular Weight | 639 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Blue Ice or 4ºC |
| Product Type | Redox Probe |
| Solubility | Soluble in DMSO |
| Source | Synthetic |
| Appearance | Orange Solid |
| Safety Phrases |
Classification: Caution: Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection |
| Cite This Product | FCR1 (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-180) |
Biological Description
| Alternative Names | 7-(Diethylamino)-N-((1r,4r)-4-(2-(10-ethyl-2,4-dioxo-4,10-dihydrobenzo[g]pteridin-3(2H)-yl)acetamido)cyclohexyl)-2-oxo-2H-chromene-3-carboxamide (FCR1) |
| Research Areas | Cancer, Oxidative Stress |
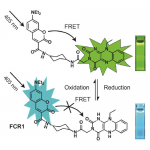
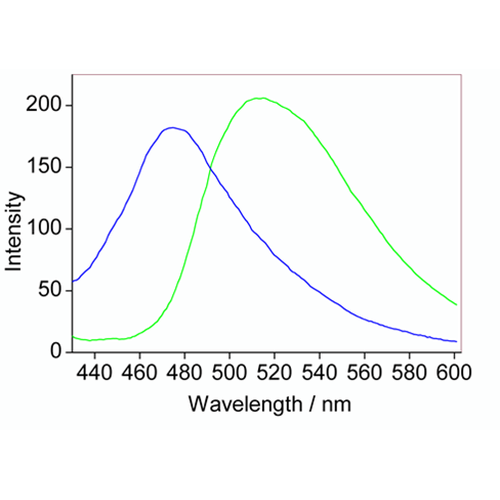
| Scientific Background | FCR1 or flavin coumarin redox sensor 1, is a novel ratiometric fluorescent redox sensor utilized in fluorescence lifetime imaging microscopy and flow cytometry. In the oxidized form, excitation of FCR1 at 405nm results in a green fluorescence with max emissions at 525nm. Treatment with a mild reducing agent (including sodium cyanoborohydride, DTT, and glutathione) reduces the flavin thereby decreasing the green fluorescence intensity, and increasing the blue. Re-oxidation can be achieved using mild oxidizing agents. In general, the FCR1 probe can be used to observe changes in oxidative capacity without interference from background effects. |
| References | 1. Kaur A., Haghighatbin M.A., Hogan C.F., and New E.J. (2015) Chem. Commun. Epub. |
Product Images
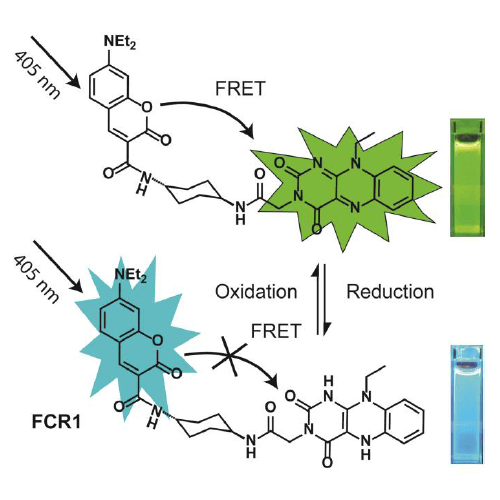
Chemical structure and design of FCR1 (SIH-180), showing FRET processes in oxidised form. Inset: photographs of cuvettes of FCR1 in oxidised and reduced forms under 365 nm excitation. Images used with permission from Kaur A, Haghighatbin MA, Hogan CF, New EJ. Chem Commun (Camb). 2015 Jun 16;51(52):10510-3.
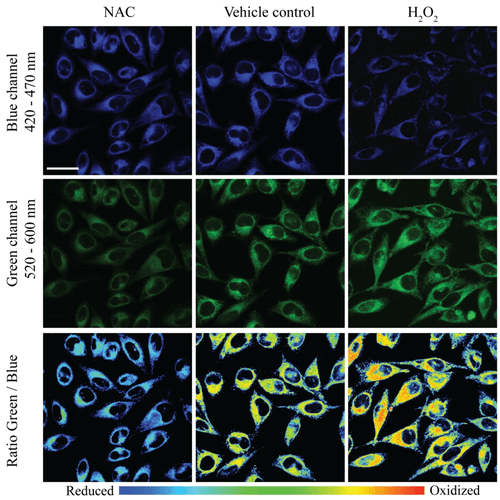
Two photon – confocal microscopy imaging of HeLa cells treated with FCR1 (SIH-180, 10 µM, 15 min, λex = 820 nm) and (a) N-acetyl cysteine (50 µM, 30 min), (b) vehicle control and (c) H2O2 (50 µM, 30 min) in blue and green channels. The pseudo colour ratio images indicate the ratio of emission intensity in the green channel to blue channel. Scale bar represents 20 µm. Images used with permission from Kaur A, Haghighatbin MA, Hogan CF, New EJ. Chem Commun (Camb). 2015 Jun 16;51(52):10510-3.
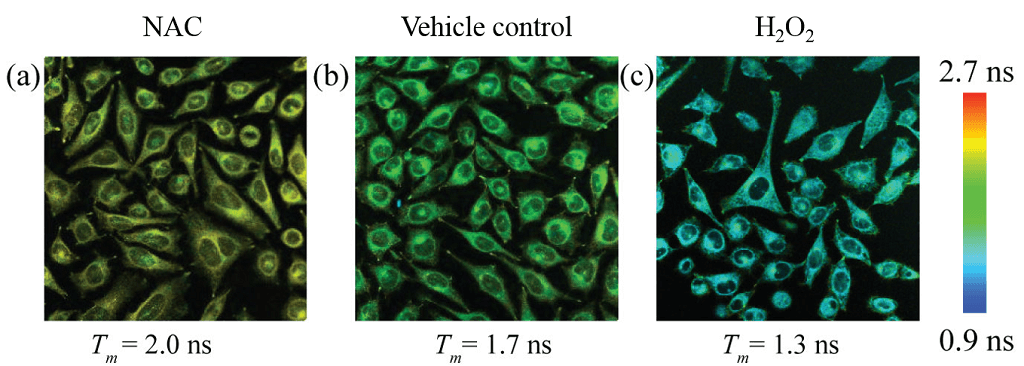
Fluorescence lifetimes of the donor (420 – 470 nm) in HeLa cells treated with FCR1 (SIH-180, 10 µM, λex = 820 nm) and N-acetyl cysteine, vehicle control and H2O2. Pseudo-colour images represent mean lifetime. Scale bar represents 50 µm. Images used with permission from Kaur A, Haghighatbin MA, Hogan CF, New EJ. Chem Commun (Camb). 2015 Jun 16;51(52):10510-3.
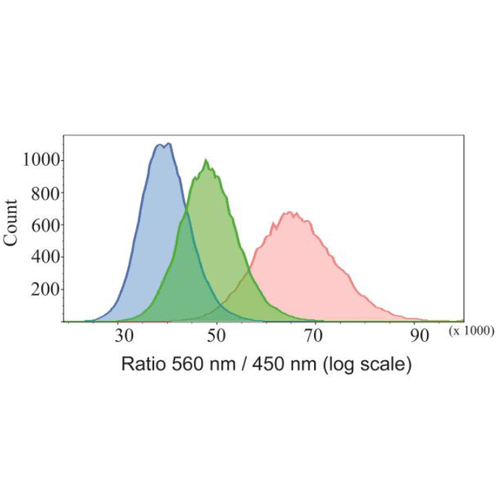
Flow cytometric studies of HeLa cells treated with FCR1 (SIH-180, 10 µM, λex = 405 nm) showing the fluorescence ratio (560 / 450 nm) when treated with N-acetyl cysteine (blue), vehicle control (green) and H2O2 (red). Images used with permission from Kaur A, Haghighatbin MA, Hogan CF, New EJ. Chem Commun (Camb). 2015 Jun 16;51(52):10510-3.





Reviews
There are no reviews yet.