| Product Name | Irinotecan HCl |
| Description |
Topoisomerase I inhibitor |
| Purity | >98% |
| CAS No. | 100286-90-6 |
| Molecular Formula | C33H38N4O6•HCl |
| Molecular Weight | 623.14 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inducer |
| Solubility | Soluble to 50 mg/ml in DMSO |
| Source | Synthetic |
| Appearance | Yellow solid |
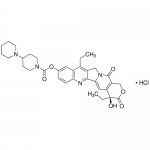
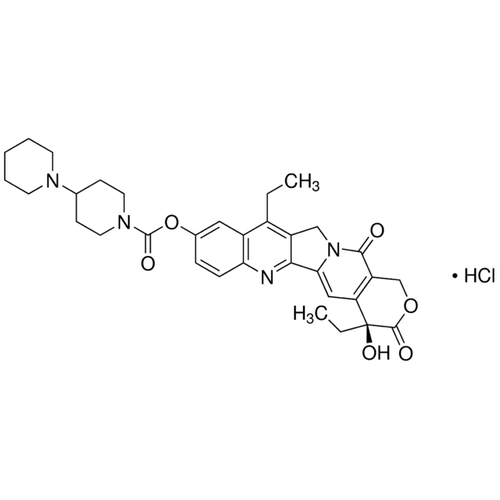
| SMILES | CCC1=C2CN3C(=CC4=C(C3=O)COC(=O)[C@@]4(CC)O)C2=NC5=C1C=C(C=C5)OC(=O)N6CCC(CC6)N7CCCCC7 |
| InChI | InChI=1S/C33H38N4O6/c1-3-22-23-16-21(43-32(40)36-14-10-20(11-15-36)35-12-6-5-7-13-35)8-9-27(23)34-29-24(22)18-37-28(29)1 |
| InChIKey | UWKQSNNFCGGAFS-XIFFEERXSA-N |
| Safety Phrases |
Classification: Harmful. May be harmful if inhaled, swallowed or absorbed through skin. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible) Risk Phrases: R20/21/22 - Harmful by inhilation, in contact with skin and if swallowed R62 - Possible risk of impaired fertility R68 - Possible risk of irreversible effects Hazard Phrases: H302 |
| Cite This Product | Irinotecan HCl (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-243) |
Biological Description
| Alternative Names | (4S)-4,11-Diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl 1,4'-bipiperidine-1'-carboxylate, CPT11, Camptothecin 11 |
| Research Areas | Apoptosis, Cancer |
| PubChem ID | 74990 |
| Scientific Background | Irinotecan is a topoisomerase 1 inhibitor, which prevents DNA from unwinding. Chemically it is a semisynthetic analogue of the natural alkaloid camptothecin. It is activated by hydrolysis to SN-38 which is then inactivated by glucuronidation by UGT1A1. This inactivation eventually leads to inhibition of both DNA replication and transcription (1, 2). |
| References |
1. Innocentri F., et al. (2004) J Clin. Oncol. 22(8): 1382-1388. 2. O’Dwyer P.J., and Catalano R.B. (2006) J Clin Oncol. 24(28): 4534-4538. |

Reviews
There are no reviews yet.