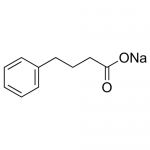
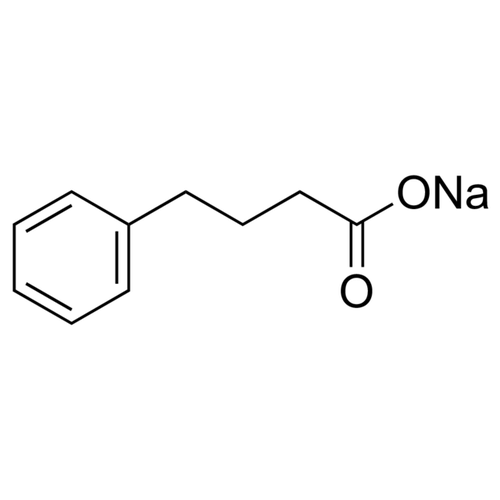
| Product Name | Phenylbutyrate Na |
| Description |
HDAC inhibitor |
| Purity | >98% |
| CAS No. | 1716-12-7 |
| Molecular Formula | C10H11NaO2 |
| Molecular Weight | 186.18 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inducer |
| Solubility | Soluble to 1100 mM in water and 100 mM in DMSO |
| Source | Synthetic |
| Appearance | White solid |
| SMILES | C1=CC=C(C=C1)CCCC(=O)[O-].[Na+] |
| InChI | InChI=1S/C10H12O2.Na/c11-10(12)8-4-7-9-5-2-1-3-6-9;/h1-3,5-6H,4,7-8H2,(H,11,12);/q;+1/p-1 |
| InChIKey | VPZRWNZGLKXFOE-UHFFFAOYSA-M |
| Safety Phrases |
Classification: Harmful. May be harmful if inhaled, swallowed or absorbed through skin. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection |
| Cite This Product | Phenylbutyrate Na (StressMarq Biosciences, Canada, Cat # SIH-255) |
Biological Description
| Alternative Names | Sodium 4-phenylbutanoate, 4-PB |
| Research Areas | Apoptosis, Cancer, Cancer Growth Inhibitors, HDAC Inhibitors |
| PubChem ID | 5258 |
| Scientific Background | Sodium phenylbutyrate is metabolized in the human body by beta-oxidation to phenylacetate, which is then conjugated with glutamine to phenylacetylglutamine, which is eliminated with urine (1). It inhibits cell proliferation, invasion and migration and induces apoptosis in glioma cells. It also inhibits protein isoprenylation, depletes plasma glutamine, increases production of fetal hemoglobin through transcriptional activation of gamma-globin gene (2). |
| References |
1. Batashaw M.L., MacArthur R.B., and Tuchman M. (2001) J Pediatr. 138: S46-54. 2. Melichar B., et al. (1998) Clin Cancer Res. 4:3069-3076. |

Reviews
There are no reviews yet.