| Product Name | Valinomycin |
| Description |
Apoptosis Inducer |
| Purity | >98% |
| CAS No. | 2001-95-8 |
| Molecular Formula | C54H90N6O18 |
| Molecular Weight | 1111.32 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inducer |
| Solubility | Soluble to 25 mM in DMSO and 25 mM in ethanol. |
| Source | Synthetic |
| Appearance | White Solid |
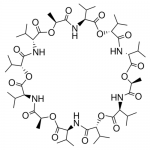
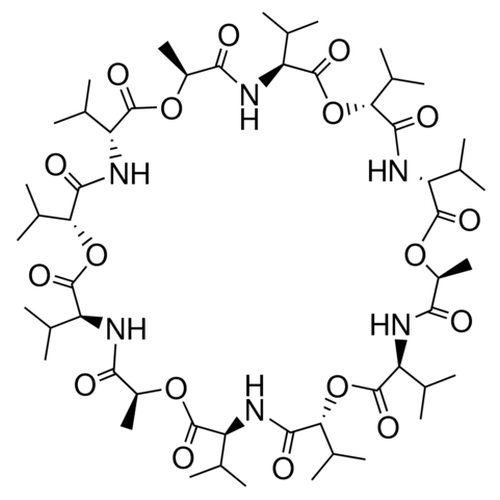
| SMILES | C[C@H]1C(=O)N[C@H](C(=O)O[C@@H](C(=O)N[C@@H](C(=O)O[C@H](C(=O)N[C@H](C(=O)O[C@@H](C(=O)N[C@@H](C(=O)O[C@H](C(=O)N[C@H](C(=O)O[C@@H](C(=O)N[C@@H](C(=O)O1)C(C)C)C(C)C)C(C)C)C)C(C)C)C(C)C)C(C)C)C)C(C)C) |
| InChI | InChI=1S/C54H90N6O18/c1-22(2)34-49(67)73-31(19)43(61)55-38(26(9)10)53(71)77-41(29(15)16)47(65)59-36(24(5)6)51(69)75-33(21)45( |
| InChIKey | FCFNRCROJUBPLU-UYBNATROSA-N |
| Safety Phrases |
Classification: Very Toxic. May be fatal if inhaled, swallowed or absorbed through skin. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible) Risk Phrases: R23/24/25 - Toxic by inhalation, in contact with skin and if swallowed R68 - Possible risk of irreversible effects Hazard Phrases: H300-H310 Precautionary Phrases: P264-P280-P302 + P350-P310 |
| Cite This Product | Valinomycin (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-230) |
Biological Description
| Alternative Names | (3R,6R,9S,12S,15R,18R,21S,24S,27R,30R,33S,36S)-3,6,9,15,18,21,27,30,33-Nonaisopropyl-12,24,36-trimethyl-1,7,13,19,25,31-hexaoxa-4,10,16,22,28,34-hexaazacyclohexatriacontane-2,5,8,11,14,17,20,23,26,29, 32,35-dodecone |
| Research Areas | Apoptosis, Cancer |
| PubChem ID | 5649 |
| Scientific Background | Valinomycin is a dodecadepsipeptide, and a member of a group of natural neutral ionophores. It is highly selective for potassium ions within the cell membrane (1). It functions as a potassium-specific transporter and facilitates the movement of potassium ions through lipid membranes down an electrochemical potential gradient (2). |
| References |
1. Lars R., Jenkins A.T.A. (2007) Bioelectrochem. 70(2): 387-393. 2. Cammann K. (1985) Top Curr Chem. 128: 219-258. |

Reviews
There are no reviews yet.