| Product Name | Axitinib |
| Description |
VEGFR kinase inhibitor |
| Purity | >98% (HPLC) |
| CAS No. | 319460-85-0 |
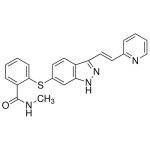
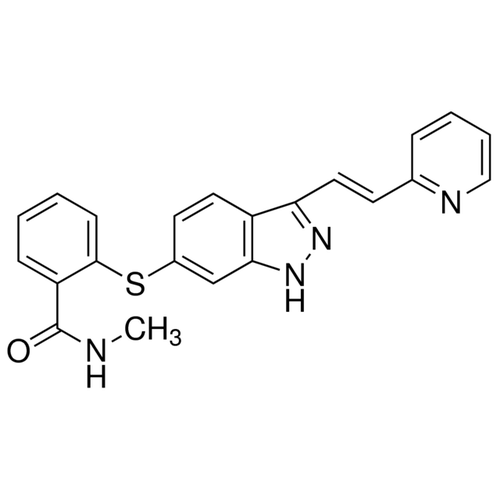
| Molecular Formula | C22H18N4OS |
| Molecular Weight | 386.5 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 25 mM in DMSO |
| Source | Synthetic |
| Appearance | White to tan powder |
| SMILES | CNC(=O)C1=CC=CC=C1SC2=CC3=C(C=C2)C(=NN3)/C=C/C4=CC=CC=N4 |
| InChI | InChI=1S/C22H18N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-14H,1H3,(H,23,27)(H,25,26)/b12-9+ |
| InChIKey | RITAVMQDGBJQJZ-FMIVXFBMSA-N |
| Safety Phrases |
Classification: Acute toxicity, Oral (Category 4), H302 Acute aquatic toxicity (Category 1), H400 Chronic aquatic toxicity (Category 1), H410 Safety Phrases: S22 - Do not breathe dust. S24/25 - Avoid contact with skin and eyes. S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection. Hazard statements: H302 Harmful if swallowed. H410 Very toxic to aquatic life with long lasting effects Precautionary statements: P264 Wash skin thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P273 Avoid release to the environment. P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell. P330 Rinse mouth. P391 Collect spillage. P501 Dispose of contents/ container to an approved waste disposal plant. |
| Cite This Product | Axitinib (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-494) |
Biological Description
| Alternative Names | AG-013736, N-Methyl-2-((3-((1E)-2-(pyridin-2-yl)ethenyl)-1H-indazol-6-yl)sulfanyl)benzamide, N-Methyl-[[3[(1E)-2-(2-pyridinyl)ethenyl]-1H-indazol-6-yl]thio]-benzamide |
| Research Areas | Apoptosis, Cancer, Cancer Growth Inhibitors, Cardiovascular System, Cell Signaling, Tyrosine Kinase Inhibitors |
| PubChem ID | 6450551 |
| Scientific Background | Axitinib is a potent and selective tyrosine kinase inhibitor that blocks VEGF receptors 1, 2 and 3. It has also been shown to inhibit PDGFR-β and c-Kit. |
| References | 1. Hu-Lowe D.D., et al. (2008) Clin. Cancer Res: J. Am. Ass. Cancer Res. 14(22): 7272–7283. |

Reviews
There are no reviews yet.