| Product Name | Entacapone |
| Description |
COMT inhibitor |
| Purity | >98% (HPLC); NMR (conforms) |
| CAS No. | 130929-57-6 |
| Molecular Formula | C14H15N3O5 |
| Molecular Weight | 305.3 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (30 mg/ml); or ethanol (3 mg/ml) |
| Source | Synthetic |
| Appearance | Yellow powder |
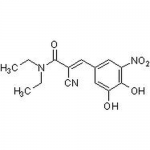
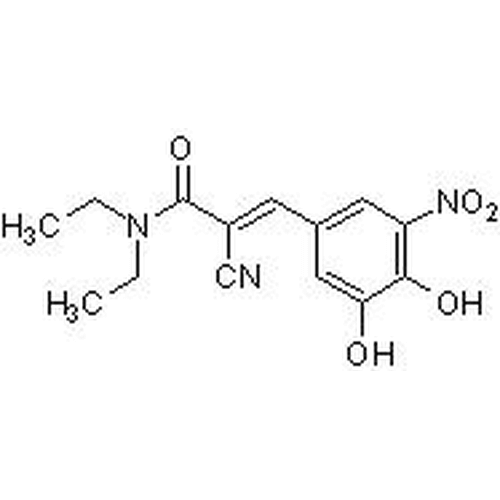
| SMILES | OC1=CC(/C=C(C#N)/C(N(CC)CC)=O)=CC([N+]([O-])=O)=C1O |
| InChI | InChI=1S/C14H15N3O5/c1-3-16(4-2)14(20)10(8-15)5-9-6-11(17(21)22)13(19)12(18)7-9/h5-7,18-19H,3-4H2,1-2H3/b10-5+ |
| InChIKey | JRURYQJSLYLRLN-BJMVGYQFSA-N |
| Safety Phrases |
Classification: Not a hazardous substance or mixture. Safety Phrases: S22 - Do not breathe dust. S24/25 - Avoid contact with skin and eyes. S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection. |
| Cite This Product | Entacapone (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-578) |
Biological Description
| Alternative Names | (2E)-2-Cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-2-propenamide |
| Research Areas | Cell Signaling, Neurodegeneration, Neuroscience, Neurotransmission, Parkinson's Disease, Synuclein |
| PubChem ID | 5281081 |
| Scientific Background | Entacapone is a selective and reversible inhibitor of the enzyme catechol-O-methyltransferase (COMT). It is used in the therapy of Parkinson's Disease as adjunctive therapy in conjuction with levodopa and carbidopa. It inhibits alpha synuclein and beta-amyloid oligomerization and fibrillogenesis. |
| References | 1. Di Giovanni S., et al. (2010) J Biol Chem. 285 (20): 14941-14954. |

Reviews
There are no reviews yet.