| Product Name | Fluoxetine HCl |
| Description |
Serotonin reuptake inhibitor |
| Purity | ≥98% (HPLC); NMR (Conforms) |
| CAS No. | 56296-78-7 |
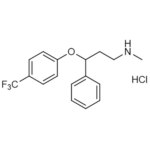
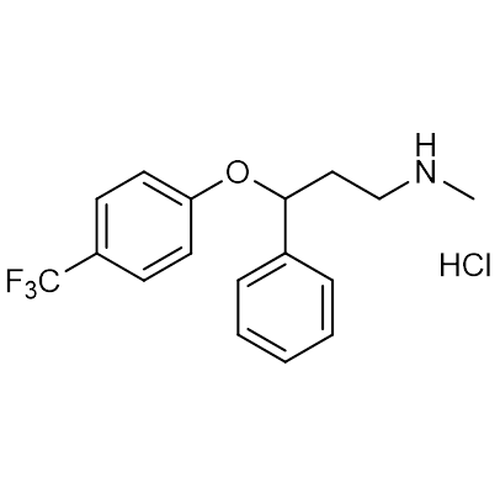
| Molecular Formula | C17H18F3NO • HCl |
| Molecular Weight | 345.8 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (35 mg/ml); or water (4 mg/ml) |
| Source | Synthetic |
| Appearance | White powder |
| SMILES | CNCCC(C1=CC=CC=C1)OC2=CC=C(C=C2)C(F)(F)F.Cl |
| InChI | InChI=1S/C17H18F3NO.ClH/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20;/h2-10,16,21H,11-12H2,1H3;1H |
| InChIKey | GIYXAJPCNFJEHY-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Danger GHS Hazard Statements, H302 (98%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (92.1%): Causes skin irritation [Warning Skin corrosion/irritation] H318 (96%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H400 (58.4%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] Precautionary Statement Codes,: P264, P264+P265, P270, P273, P280, P301+P317, P302+P352, P305+P354+P338, P317, P321, P330, P332+P317, P362+P364, P391, and P501 |
| Cite This Product | Fluoxetine HCl (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-631) |
Biological Description
| Alternative Names | (±)-N-Methyl-γ-[4-(trifluoromethyl)phenoxy]benzenepropanamine hydrochloride; LY-110,140 |
| Research Areas | Neurodegeneration, Neuroscience, Neurotransmission, Stem Cells, Neural Stem Cells |
| PubChem ID | 62857 |
| Scientific Background | A selective serotonin reuptake inhibitor (SSRI) with high selectivity for the serotonin transporter (Kd=0.81 nM) over the norepinephrine (Kd=240 nM) and dopamine (Kd=3.6 M) transporters(1). A clinically useful antidepressive agent (2). Has shown some potential in autism spectrum disorders (3). Attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage (4). Orally active and active in vivo. Increases proliferation of neuronal precursors derived from human embryonic stem cells, inducing differentiation and strongly enhancing neuronal characteristics (5). |
| References |
1. M Tatsumi et al. Eur. J. Pharmacol. 1997 340:249 2. P Benfield et al. Drugs 1986 32:481 3. A Benvenuto et al. Brain Dev. 2013 35:119 4. FY Liu et al. J. Neuroinflammation 2018 15:347 5. EA Chang et al. Int. J. Dev. Biol. 2010 54:707 |

Reviews
There are no reviews yet.