| Product Name | Gemcitabine HCl |
| Description |
DNA polymerase inhibitor |
| Purity | >98% (HPLC); NMR (conforms) |
| CAS No. | 122111-03-9 |
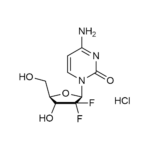
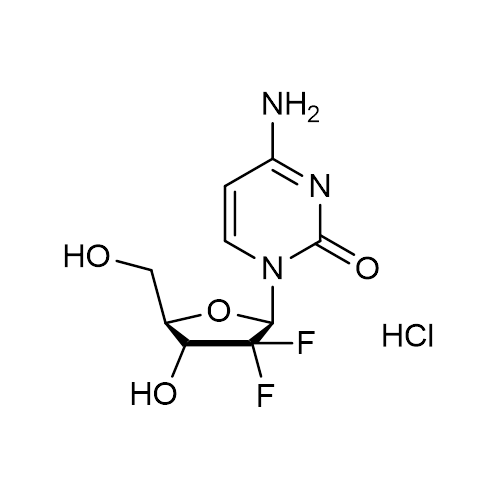
| Molecular Formula | C9H11F2N3O4 • HCl |
| Molecular Weight | 299.7 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (20 mg/ml); or Water (25 mg/ml) |
| Source | Synthetic |
| Appearance | Off-white powder |
| SMILES | C1=CN(C(=O)N=C1N)C2C(C(C(O2)CO)O)(F)F.Cl |
| InChI | InChI=1S/C9H11F2N3O4.ClH/c10-9(11)6(16)4(3-15)18-7(9)14-2-1-5(12)13-8(14)17;/h1-2,4,6-7,15-16H,3H2,(H2,12,13,17);1H/t4-,6-,7-;/m1./s1 |
| InChIKey | OKKDEIYWILRZIA-OSZBKLCCSA-N |
| Safety Phrases | Classification: Danger. Hazard Statements: H360. Precautionary Statements: P201 - P202 - P280 - P308 + P313 - P405 - P501 |
| Cite This Product | Gemcitabine HCl (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-623) |
Biological Description
| Alternative Names | 2′-Deoxy-2′,2′-difluorocytidine hydrochloride; dFdC |
| Research Areas | Cancer, Cell Signaling, DNA Synthesis, DNA/RNA, Epigenetics and Nuclear Signaling, Topoisomerases |
| PubChem ID | 60749 |
| Scientific Background | Gemcitabine HCl is a nucleoside analog used as an anticancer agent. It inhibits DNA polymerase and ribonucleotide reductase, leading to apoptosis. While primarily studied in oncology, its mechanisms of DNA synthesis inhibition and cell cycle arrest are relevant to neuroscience research, particularly in models of cytotoxic stress, DNA damage response, and neurodegeneration. |
| References |
1. Mini E., et al. (2006) Ann. Oncol. 17 Suppl 5:v7. 2. Heinemann V., et al. (1995) Semin. Oncol. 22(4 Suppl 11):11. 3. Heinemann V., et al. (1992) Cancer Res. 52:533. 4. Pourquier P., et al. (2002) Clin. Cancer Res. 8:2499. |

Reviews
There are no reviews yet.