| Product Name | Genistein |
| Description |
Tyrosine kinase inhibitor |
| Purity | >98% (HPLC) |
| CAS No. | 446-72-0 |
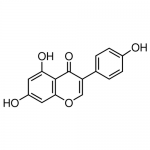
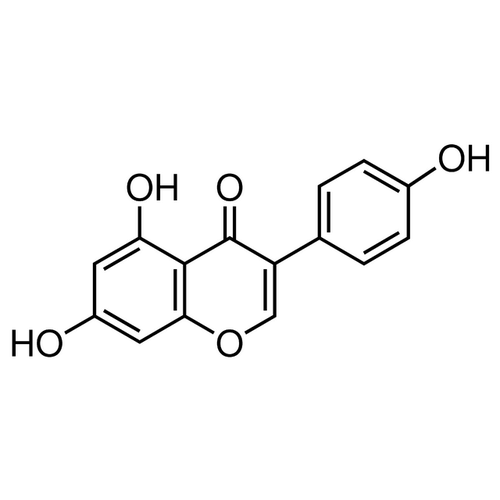
| Molecular Formula | C15H10O5 |
| Molecular Weight | 270.2 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble in DMSO (30 mg/ml), 100% ethanol (4 mg/ml) or dilute aqueous base; insoluble in water |
| Source | Synthetic |
| Appearance | Creamy off-white solid |
| SMILES | C1=C(O)C=C(C2=C1OC=C(C2=O)C3=CC=C(O)C=C3)O |
| InChI | InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-13-6-10(17)5-12(18)14(13)15(11)19/h1-7,16-18H |
| InChIKey | TZBJGXHYKVUXJN-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Acute toxicity, Oral (Category 4), H302 Safety Phrases: S22 - Do not breathe dust. S24/25 - Avoid contact with skin and eyes. S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection. Hazard statements: H302 Harmful if swallowed Precautionary statements: P264 Wash skin thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell. P330 Rinse mouth. P501 Dispose of contents/ container to an approved waste disposal plant. |
| Cite This Product | Genistein (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-446) |
Biological Description
| Alternative Names | 4',5,7-Trihydroxyisoflavone, 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, 4′,5,7-Trihydroxyisoflavone, 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one |
| Research Areas | Apoptosis, Cancer, Cancer Growth Inhibitors, Cell Signaling, Tyrosine Kinase Inhibitors |
| PubChem ID | 5280961 |
| Scientific Background | Genistein has a wide range of biological actions. It is an inhibitor of tyrosine protein kinase, including epidermal growth factor receptor kinase. It also inhibits DNA topoisomerase and α-glucosidase. It produces cell cycle arrest and apoptosis, and inhibits endocytosis, as well as, inhibits insulin-induced glucose uptake in adipocytes. |
| References |
1. Akiyama T., et al. (1987) J Biol. Chem. 262(12): 5592–5595. 2. Bazuine M., van den Broek P.J.A., & Maassen J. A. (2005) Biochem. Biophys. Res. Comm. 326(2): 511–514. 3. Linassier C., Pierre M., Le Pecq J.B., & Pierre J. (1990) Biochem. Pharm. 39(1): 187–193. 4. Dean N.M., Kanemitsu M., & Boynton A.L. (1989) Biochem. Biophys. Res. Comm. 165(2): 795–801. |

Reviews
There are no reviews yet.