| Product Name | J9 |
| Description |
Glucocorticoid resistance inhibitor |
| Purity | 99.90% |
| Molecular Formula | C12H12N4 |
| Molecular Weight | 212.26 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Blue Ice or 4ºC |
| Product Type | Inhibitor |
| Solubility | Soluble in CDCI3 |
| Source | Synthetic |
| Appearance | White Solid |
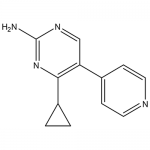
| SMILES | NC1=NC(C2CC2)=C(C3=CC=NC=C3)C=N1 |
| InChI | InChI=1S/C12H12N4/c13-12-15-7-10(8-3-5-14-6-4-8)11(16-12)9-1-2-9/h3-7,9H,1-2H2,(H2,13,15,16) |
| InChIKey | PQLYPFZAOUDDHB-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Caution: Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection |
| Cite This Product | J9 (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-182) |
Biological Description
| Alternative Names | J9,4-cyclopropyl-5-(pyridin-4-yl)pyrimidin-2-amine |
| Research Areas | Cancer, Cell Signaling |
| PubChem ID | PMC4094258 |
| Scientific Background | J9, in combination with dexamethasone inhibits cell growth in T-cell acute lymphoblastic leukemia (T-ALL) through the upregulation of the glucocorticoid receptor. Patients can develop glucocorticoid resistance rendering the treatment ineffective. J9 and its mechanism of action provides a useful strategy for overcoming the resistance. The EC50 of J9 in combination with dexamethasone is 28 uM. J9 alone was less toxic. |
| References | 1. Cantley A.M., et al. (2014) ACS Medicinal Chemistry Letters 5.7: 754-759. |


Reviews
There are no reviews yet.