| Product Name | Lovastatin |
| Description |
HMG-CoA reductase inhibitor |
| Purity | >98% |
| CAS No. | 75330-75-5 |
| Molecular Formula | C24H36O5 |
| Molecular Weight | 404.54 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 50 mM in ethanol and 100 mM in DMSO |
| Source | Synthetic |
| Appearance | White solid |
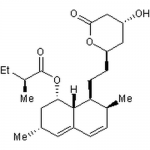
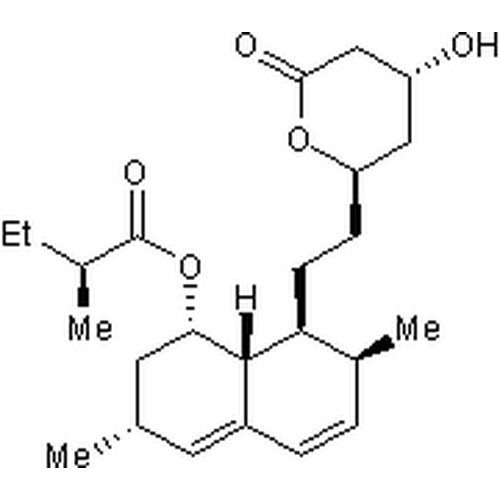
| SMILES | CC[C@H](C)C(=O)O[C@H]1C[C@H](C=C2[C@H]1[C@H]([C@H](C=C2)C)CC[C@@H]3C[C@H](CC(=O)O3)O)C |
| InChI | InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18 |
| InChIKey | PCZOHLXUXFIOCF-BXMDZJJMSA-N |
| Safety Phrases |
Classification: Harmful. May be harmful if inhaled, swallowed, or absorbed through skin. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection Risk Phrases: R62- Possible risk of impaired fertility |
| Cite This Product | Lovastatin (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-256) |
Biological Description
| Alternative Names | (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydro-1-naphthalenyl (2S)-2-methylbutanoate, Mevinolin |
| Research Areas | Apoptosis, Cancer |
| PubChem ID | 53232 |
| Scientific Background | Lovastatin is a member of the drug class of statins, used for lowering cholersterol, thereby preventing cardiovascular disease. They have a powerful inhibitory effect on HMG-CoA reducatse (1). |
| References | 1. Alberts A.W. (1998) Am J Cardio. 62(15): 10J-15J. |

Reviews
There are no reviews yet.